Lecture 17. Enthalpy of reaction
Tuesday 26 March 2024
Measuring changes in enthalpy (ΔH) using constant pressure calorimetry. Thermochemical equations. Using bond enthalpies to estimate enthalpies of reaction. Using standard enthalpies of formation to calculate enthalpies of reaction.
Reading: Tro NJ. Chemistry: Structure and Properties (3rd ed.) - Ch.9, §9.9 & §9.10 (pp.406-415).
Summary
We finish our treatment of thermochemistry by defining and learning the rules for manipulation of thermochemical equations. What our text refers to as Hess's law (see Ref.1, pp.387-388) is a succinct summary of the rules for treating the reaction enthalpy changes (ΔH values) of the thermochemical equations under manipulation. We then consider and demonstrate two methods for obtaining values for the standard enthalpy change of a reaction. The two methods are the use of tabulated bond enthalpies, and the use of tabulated standard enthalpies of formation.
Standard enthalpies of formation and their use
Thermodynamic quantities known as standard enthalpies of formation have been measured for a great many compounds and are used in a procedure to calculate the enthalpy of any reaction involving these compounds. The standard enthalpy of formation (ΔH°f) of a compound is defined as the heat absorbed or released when one mole of the compound is formed from its elements in their most stable states. The temperature is usually specified as 298 K. The standard enthalpy of formation of the most stable form of an element at the specified temperature is zero by definition. ΔH°f values are usually found in an appendix of a general chemistry textbook.
The procedure to calculate the standard enthalpy change (ΔH°rxn) for a generalized chemical reaction,
aA + bB + cC + ···· → xX + yY + zZ + ····
is to combine the ΔH°f values for the products and reactants according to the formula
ΔH°rxn = x ΔH°f, X + y ΔH°f, Y + z ΔH°f, Z + ···· − a ΔH°f, A − b ΔH°f, B − c ΔH°f, C − ····
This formula, based on the principle of Hess's law, is sometimes referred to as Hess's law equation. It should be noted that the stoichiometric coefficient could be interpreted as introducing the unit mol to each of the ΔH°f values, given in kJ mol−1, so that the ΔH°rxn we compute is an extensive quantity. Alternatively, we can interpret the value intensively (keeping the stoichiometric coefficients unitless) as the amount of heat released or absorbed per mol of the reaction as written.
Bond enthalpy
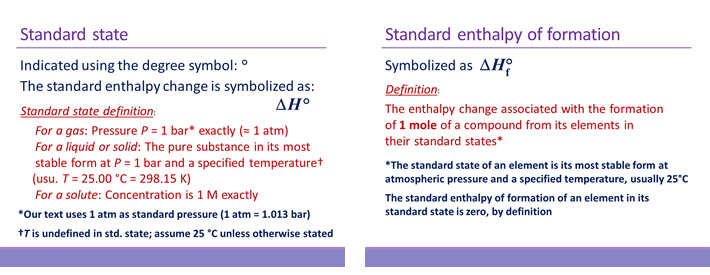
Bond enthalpy is the enthalpy change associated with breaking a particular bond in 1 mol of gas-phase molecules. By convention, bond enthalpies are reported as positive values, corresponding to the endothermic process of bond breakage. Bond enthalpies (or bond energies) tabulated for common types of bonds can be used to estimate the enthalpy change of many different reactions. Note that only for diatomic molecules can the values be determined exactly. For polyatomic molecules, bond enthalpies are average values.
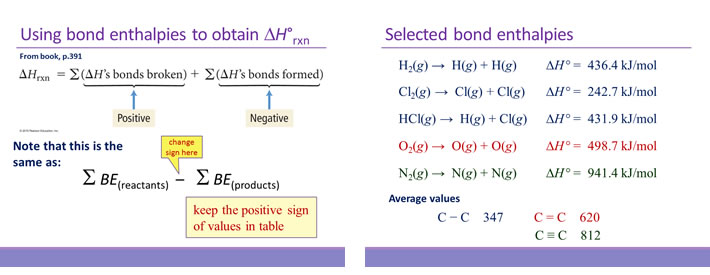
In using bond enthalpies to estimate a ΔH°rxn, the procedure is to sum all the bond enthalpies of reactants and subtract from this the sum of all the bond enthalpies of the products.