BIOCHEMISTRY TOPICS
Fatty acids
Fatty acid structure and nomenclature. Biological roles of fatty acids.
Fatty acids are primary constituents of lipds (fats). Fatty acids contain a long aliphatic (hydrocarbon) chain, with a carboxyl (-COOH) group at one end, which is the acidic moeity. The aliphatic group may be fully saturated, in which case the fatty acid is said to be saturated, or it may contain one or more double bonds, in which case it is termed an unsaturated fatty acid. At physiological pH, the carboxyl group of a fatty acid is in its ionized, carboxylate form. This anionic (negatively-charged) form is represented in the structures of fatty acids shown here. The structure of a saturated fatty acid can be represented in abbreviated form as R-COO−, with R representing CH3(CH2)n and the total number of carbon atoms is n + 2. Typically, the total number of carbons is 10-20 or so, most commonly 16, in which case the compound is called palmitate (shown below), or 18, giving stearate. The unionized forms of these fatty acids are palmitic acid and stearic acid, respectively.
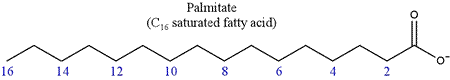
In numbering of fatty acids, the carbon of the carboxyl group is assigned the number 1. Most naturally-occurring fatty acids have an even number of carbon atoms. An unsaturated fatty acid contains one or more double bonds between carbon atoms in its hydrocarbon chain. The generic formula for an unsaturated fatty acid with only one double bond is R-COO–, with R representing the chemical grouping CH3(CH2)mCH=CHCH2(CH2)n. If m=n=7, this would represent the 18-carbon unsaturated fatty acid oleate (oleic acid). The configuration of the double bonds in naturally-occurring unsaturated fatty acids is nearly always cis.
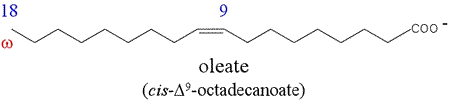
A polyunsaturated fatty acid is one that contains more than one double bond. Linoleate is a prototypical polyunsaturated fatty acid that contains an unbranched chain of 18 carbon atoms. It is a so-called omega 6 (ω-6) fatty acid, and is of importance in human nutrition due to the fact that it cannot be synthesized by mammals and must therefore be included in their diet. Linoleate is hence regarded as an essential fatty acid. The importance of linoleate derives largely from its role as a precursor of arachidonate.
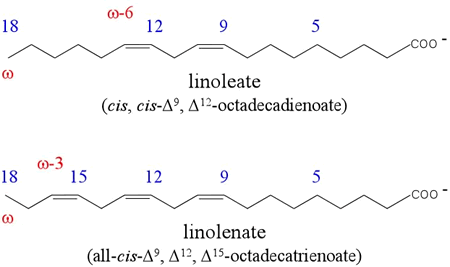
The figure at left illustrates nomenclature of unsaturated fatty acids. The numbering begins at the carboxylate group (on the right) - its carbon atom is numbered 1. Atom numbers increase leftward. The positions of double bond(s) are indicated by the Δ (delta) notation. The superscript of the Δ is that of the lowest numbered carbon atom of the double bond. Stereochemistry (cis or trans) is indicated explicitly. The ω (omega) notation - focuses on the position of the double bond furthest from the carboxylate group.