Lecture 7. Periodic trends for atoms and ions
Tuesday 6 February 2024
Electron configurations for ions. Ionization energy. Periodic trends in ionization energy and ionic radii. Electron affinities, metallic character, and their general periodic trends.
Reading: Tro NJ.Chemistry: Structure and Properties (3rd ed.) - Ch.3, §3.7-3.9 (pp.142-155)
Summary
Ionization energy
The ionization energy (abbreviated as IE) of an element is expressed as the energy (usually in units of kilojoules, kJ) required to remove an electron from each of a mole of atoms or ions. What specifically is called the first ionization energy (IE1) can be represented by the chemical equation
X(g) → X+(g) + e−
The general periodic trends in ionzation energies can be understood in terms of the periodic trends in Zeff, but the details of how ionzation energies vary depends on electronic configurations.
Electron affinity
The electron affinity (EA) for an element is defined as the magnitude of energy (in kJ/mol) released by the process represented by the chemical equation
X(g) + e− → X−(g)
Similar to atomic radii and ionzation energies, electron affinities show periodic trends, although they are less clear cut that the former trends. Excepting the noble gas elements of Group 8A, the highest EA values are associated with the nonmetals on the right side of the periodic table. The lowest values are seen for metals on the left side. The values of EA generally increase from left to right along a period, but there are striking exceptions. Again the broad trends can be related to the periodic trend in Zeff. A detailed account of how electron affinity varies requires consideration of the electronic configurations of the neutral atom and the anionic form that results by addition of an electron.
It is important to note the convention for the sign of the energy changes associated with processes such as ionization and neutral atoms gaining electrons. The amount of energy consumed in an energy-requiring, or endothermic process is, by chemical convention, given a positive sign. Since ionization requires an input of energy, ionization energies are always positive. For most elements, the equation corresponding to EA represents an energy-releasing, or exothermic process. The amount of energy released in an exothermic process is conventionally given a negative sign.
Isoelectronic series
A series of species with the same number of electrons is called an isoelectronic series. An example of an isoelectronic series that includes monatomic main group ions is shown below.
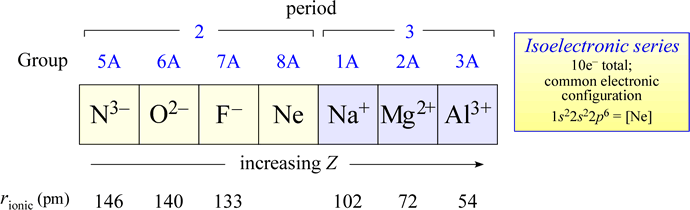
Note that the ionic radii will decrease for this series with increasing nuclear charge Z.