Lecture 8. Molecules and compounds
Tuesday 13 February 2024
Atoms, molecules and the types of chemical bonds. Chemical formulas and molecular models. Lewis notation for valence electrons and chemical bonds. Lewis structures: Symbolic representation of atoms, molecules, and polyatomic ions. Ionic bonding. Formulas and names for ionic compounds.
Reading: Tro NJ. Chemistry: Structure and Properties (3rd ed.) - Ch.4, §4.1-4.6 (pp.167-184t)
Summary
We have reached the point in our "atoms first" approach where we are ready to consider the chemical properties of the elements in a systematic way, and to develop the atomic scale models that account well for the ways in which elements are observed to combine in the macroscopic realm. Compounds are combinations of elements in characteristic fixed ratios, as measured by mass. How do the atoms arrange themselves in compounds, and how do these arrangements explain the stability and fixed proportions of the elements? We will now see how our knowledge of atomic theory enables us to understand the nanoscale features of chemical bonding.
American chemist Gilbert Newton Lewis (1875-1946) played a foundational role in the development of a modern understanding of chemical bonding. He advanced the idea that in bonding, atoms acquire a stable, noble-gas electron configuration, and introduced a symbolic representation of valence electrons that we still use today.
Symbolic and structural representations of molecules and compounds
Chemistry is rich with symbolic language, and we consider here some important examples. Our study of chemistry will lead us to become familiar with them and we'll learn to use and write chemical formulas and equations ourselves. The type of representation we choose will depend on the information we want to convey. Illustrated below are examples of four common types of molecular representations for the simple molecule water. The simplest chemical formula for a compound gives the smallest whole-number ratios of the atoms of each element that makes up that compound. This is defined as the empirical formula for a compound. If a compound can be best described as composed of molecules, a molecular formula specifies the number of each type atom that makes up the molecule. In other words, a molecular formula gives the atomic composition of a molecule, while an empirical formula specifies the simplest stoichiometry for the atoms in a compound. The word "stoichiometry" refers to the numerical relationships among chemical amounts or entities. These formulas include the atomic symbol of each type of atom in a molecule (or each element of a compound), with subscripts indicating the number atoms of each type there are, i.e. the formula stoichiometry. In the case of water, the molecular and empirical formulas are the same. They tell us that each water molecule consists of two hydrogen atoms and one oxygen atom (molecular formula) and that in every mole of water, there are 2 mol hydrogen and 1 mol oxygen. Furthermore, as it is the case that in any sample of water, the chemical amount (i.e. amount in mol or some decimal multiplier of the unit mol) of hydrogen is twice that of oxygen, the total mass of oxygen in any sample of water will be only eight times the mass of hydrogen (rather than the 16:1 O:H atomic mass ratio). This is the stoichiometric basis of the law of fixed proportions. In contrast, the molecular formula can be quite different from the empirical formula in many cases. Another compound formed between hydrogen and oxygen is hydrogen peroxide, which has the molecular formula H2O2. Since the ratio hydrogen atoms to oxygen atoms in this formula is 1:1, the empirical formula of hydrogen peroxide is HO.
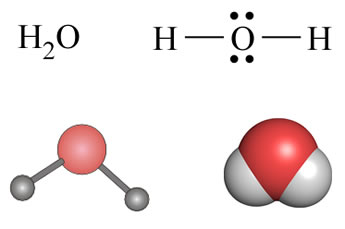
While molecular formulas are simple and easy to write within a line of normal text, they provide no explicit information about how the atoms are connected or the three-dimensional shape of the molecule.
Next is the structural, or "dash" formula in which the atoms (strictly speaking, the nuclei along with core electrons) are represented by their atomic symbols, and the lines connecting them denote chemical bonds. Such representations show connectivity between atoms, but since they are two dimensional, they do not explicitly convey three-dimensional molecular shapes. When a structural formula is written to also explicitly represent all valence electrons in a molecule (i.e., including nonbonding electrons), this is referred to as a Lewis structure. As we will see, a Lewis structure allows us to infer the (usually approximate) three-dimensional shape of a molecule.
The latter two representations are explicitly three dimensional, and they are meant to be accurate models of how the atoms are arranged in space. A ball-and-stick model - typically constructed using a molecular model kit or visualized using computer graphics - clearly shows the geometry of how the atoms are bonded. The balls represent the atomic centers in the molecule and the sticks the bonds between them. Space filling models that represents the size and shape of the molecule by showing the atoms according to appropriate definition of their sizes.