Lecture 15. Reactions and stoichiometry in solution
Tuesday 19 March 2024
Solutions and solution stoichiometrry. Molecular electrolytes: Acids, bases, and gases. Oxidation-reduction ("redox") reactions.
Reading: Tro NJ. Chemistry: Structure and Properties (3rd ed.) - Ch.8, §8.6-§8.9 (pp.352-368).
Summary
After a review of solution stoichiometry and reaction types so far, a more in depth treatment follows, adding the important oxidation-reduction reaction type to a classification scheme for chemical reactions. A preview of the calorimetry lab, which applies the principles of chemical thermodynamics, concludes the live lecture topics.
Solution stoichiometry
With the use of molarity and the stoichiometry of a balanced chemical reaction, the calculations necessary to solve the limiting reactant and theoretical yield problems. Both acid-base and precipitation reaction types provide examples of a quantitative analysis of a reaction in solution.
Types of chemical reactions
In Chapter 8 of our textbook (Ref.1), an extensive amount of chemistry is introduced, and we will attempt to address the various topics while still keeping a big picture in focus. Our overall goal is to understand the types of processes and reactions that occur in aqueous solutions. We'll introduce the three types of chemical reactions: precipitation, acid-base, and oxidation-reduction reactions.
Precipitation reactions
A precipitation reaction occurs upon the mixing of two solutions of ionic compounds when the ions present together in the mixture can form an insoluble compound. In such cases, the solution turns visibly cloudy, a phenomenon known as precipitation. The cloudiness is due to the formation of small aggregations of solid substance (the precipitate). The precipitate can be separated from the remaining solution by filtration.
Acid-base reactions
The first and simplest definition of an acid is the Arrhenius definition, which states that an acid is a substance that creates or consists of hydrogen ions in water, H+(aq). The corresponding Arrhenius definition of a base is a substance that creates or consists of hydroxide ions in water, OH–(aq).
There are seven common strong acids that dissociate completely to H+(aq) and a conjugate anion in water. Other acids dissociate only partially - these are the weak acids. A primary example of a weak acid is the common organic acid acetic acid (ethanoic acid). Vinegar contains acetic acid at a low concentration. The formula for acetic acid is typically written as CH3COOH, with the acidic hydrogen listed last. Examples of strong bases are the soluble metal hydroxides, the most common being sodium hydroxide (NaOH) and potassium hydroxide (KOH). Our primary example of a weak molecular base is ammonia, NH3. When dissolved in water, a small proportion of the ammonia molecules react to form ammonium and hydroxide ions.
Acids and bases have long been known to be chemical opposites, as they undergo neutralization reactions. We will consider examples of neutralization reactions. An acid-base titration is a quantitative, stoichiometric measurement of a neutralization reaction.
Acid-base neutralization
Our primary example of a strong acid is hydrochloric acid, HCl(aq). Hydrochloric acid forms from the dissolution of hydrogen chloride, a heterodiatomic gas, into water:
HCl(g) → HCl(aq)
As a strong acid, hydrochloric acid is a strong electrolyte. This is represented by the equation
HCl(aq) → H+(aq) + Cl−(aq)
Note that like the strong electrolyte sodium chloride, HCl converts completely to ions in water. But like acetic acid, HCl(aq) actually reacts with water, as indicated by the production of hydronium ion (abbreviated as H+(aq)). The strong base, sodium hydroxide NaOH, is an ionic compound that is highly soluble in water and completely dissociates into its component ions:
NaOH(aq) → Na+(aq) + OH−(aq)
The mixing together of solutions of hydrochloric acid and sodium hydroxide results in an acid-base neutralization reaction. A series of chemical equations below illustrate the several forms reactions involving ionic species can be represented.
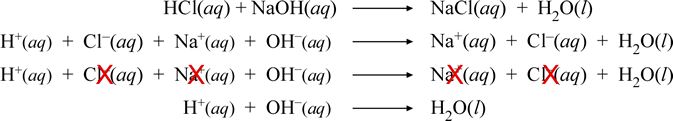
Let's look at this series, each equation in turn. The first equation is the so-called "molecular" or formula equation. The second equation is the complete ionic equation. Next, the full ionic equation is repeated, but cancellation of species appearing the same on both sides is indicated, which leads to the final equation, the net ionic equation. For the neutralization reaction between any monoprotic strong acid and strong base, the resulting net ionic equation will be the same as that shown above for HCl and NaOH.
The keys to writing correct ionic equations are to recognize the following:
• Strong electrolytes (represented as completely dissociated ions), comprised of the following:
• soluble ionic compounds, including metal hydroxides, which are strong bases,
• strong acids
• Weak electrolytes - their unionized forms normally predominate. These include:
• the weak base ammonia (NH3)
• the weak acid acetic acid (HC2H3O2
• Reactions that form precipitates, water, and gases (such as CO2).
When solutions of different ionic compositions are mixed, we will predict reactions based upon chemical knowledge, such as that water will form whenever H+(aq) and OH−(aq) are mixed together, and water and carbon dioxide form when H+(aq) and CO32−(aq) or H+(aq) and HCO3−(aq) are mixed together. We also rely upon solubility rules that we use to predict whether an insoluble combination of ions forms in a precipitation reaction.