GENERAL CHEMISTRY TOPICS
Electrolytes
Dissociation of ionic compounds in water results in the formation of mobile aqueous ionic species. Chemical equations for dissolution and dissociation in water. Strong and weak electrolytes.
Electrolytes (musical accompaniment to this topic) are substances that create ionic species in aqueous solution. We can demonstrate the existence of charge carriers in solution by means of a simple experiment. The conductivity of aqueous media can be observed by using a pair of electrodes, connected to a voltage source, that we immerse in the solution. The current the solution conducts then can be readily measured; we use a light bulb as a visual indicator of the conductivity of a solution.
When this experiment is performed with pure water, the light bulb does not glow at all. Water itself does not conduct electricity easily; it is an example of a molecular substance that is a nonelectrolyte. This is true for many other molecular substances. When soluble nonelectrolyte molecular solutes are mixed with bulk water as solvent, the light bulb still does not light up. These molecular compounds apparently do not produce ions in any significant quantity as solutes in an aqueous solution. For example, table sugar (sucrose, C12H22O11) is quite soluble in water, but a sugar solution apparently conducts electricity no better than just water alone. On the other hand, when we perform the experiment with a freely soluble ionic compound like sodium chloride, the light bulb glows brightly.
Let us represent what we think is going on with these contrasting cases of the dissolution of a molecular and an ionic compound by writing the following chemical equations:
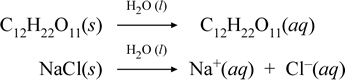
The first equation above represents the dissolution of a nonelectrolyte, the molecular compound sucrose. The second equation represents the dissolution of an ionic compound, sodium chloride. The key distinction between the two chemical equations in this case is the formation in the latter of aqueous ionic species as products. The ions are free to diffuse individually in a homogeneous mixture, and when a voltage is applied, the ions will move according to the electric potential energy difference between electrodes, thus carrying electric current. Note that water is not shown on the reactant side of these equations, but instead is shown above the arrow, indicating that water determines the environment in which the dissolution process occurs. The superstoichiometric status of water in this symbolism can be read as a dissolution process occurring with water as the solvent. We will not write water as a reactant in the formation of an aqueous solution by a simple dissolution process. There are many cases in which a substance reacts with water as it mixes with and dissolves in water. This reaction of a solute in aqueous solution gives rise to chemically distinct products. In such cases water can be explicitly shown in the chemical equation as a reactant species.
Strong and weak electrolytes
It turns out that when a soluble ionic compound such as sodium chloride undergoes dissolution in water to form an aqueous solution consisting of solvated ions, the rightward arrow used in the chemical equation is justified in that (as long as the solubility limit has not been reached) the solid sodium chloride added to solvent water completely dissociates. In other words, effectively there is 100% conversion of NaCl(s) to Na+(aq) and Cl−(aq). As a result, in our conductivity experiment, a sodium chloride solution is highly conductive due to the abundance of ions, and the light bulb glows brightly. In such a case, we say that sodium chloride is a strong electrolyte.
In contrast, consider the molecular substance acetic acid, HC2H3O2. When acetic acid is dissolved in water, it forms an undissociated, solvated, molecular species symbolized as HC2H3O2(aq), similar to the case with sucrose above. However, when we perform our conductivity test with an acetic acid solution, we find that the light bulb glows, albeit rather weakly compared to the brightness observed for the sodium chloride solution. In this case, there must be at least partial formation of ions from acetic acid in water. A chemical equation representing this process must show the production of ions. A reasonable proposal for such an equation would be:
Two things are important to note here. First, this is a case where we include water as a reactant. The two molecular substances, water and acetic acid, react to form the polyatomic ions hydronium and acetate. The equation representing this is an ionic equation. The second feature that merits further discussion is the replacement of the rightward arrow with the double single-barbed arrows symbol, signifying a chemical equilibrium and in this case the equilibrium condition for the reaction favors the reactants, meaning that in an aqueous solution of acetic acid, most of the acetic acid remains as acetic acid molecules, with only a small proportion at any time haven given up H+ to water to form the ions. The small number of ions produced explains why the acetic acid solution does not conduct electricity as well as the sodium chloride solution, resulting in only a weak illumination of the light bulb of our conductivity detector. We therefore make a distinction between strong electrolytes, such as sodium chloride, and acetic acid, which is an example of a weak electrolyte.
As the name acetic acid suggests, this substance is also an acid, as well as a weak electrolyte. Accordingly, we classify acetic acid as a weak acid. Our first (and least general) definition of an acid is a substance that creates hydronium ion in water, which is just what our ionic equation above shows, bearing in mind that a weak acid creates relatively small amounts of hydronium ion.
Chemists are very fond of abbreviations, and an important abbreviation for hydronium ion is H+(aq), and this is commonly used. This is shown in the abbreviated version of the above equation which is shown just below. To be clear, H+ itself would be just an isolated proton (for 1H); thus it is also important to note that no such species exists in aqueous solution.
Furthermore, the arrows have been made of unequal length to indicate the reactant-favored equilibrium, in which there are much fewer ions than acetic acid molecules.
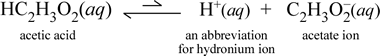
By representing hydronium as H+(aq), the ionic equation for acetic acid in water is formally balanced without including a water molecule as a reactant, which is implicit in the above equation.
Ammonia: An example of a weak electrolyte that is a weak base
Acetic acid as we have just seen is a molecular compound that is weak acid and electrolyte. Ammonia, NH3, another simple molecular compound, also reacts to a small extent with water, forming ammonium and hydroxide ions. Our first, least general definition of a base is a substance that creates hydroxide ions in water. Thus, ammonia is a weak base, and like acetic acid, does not conduct electricity nearly as well as aqueous salt. So ammonia is a weak electrolyte as well.
The symbolism of our chemical equation again indicates a reactant-favored equilibrium for the weak electrolyte.
Extensions and connections
A more quantitative approach to equilibria uses weak acids and weak bases as important examples.