GENERAL CHEMISTRY TOPICS
Chemical equations
The symbolic language of chemistry. Types of chemical equations. Balancing chemical equations.
A chemical equation is a symbolic representation of a chemical change or process. In the figure below, an example chemical equation is labeled according to its components. The set of species on the left side of the rightward arrow are called the reactants; those on the right side are the products. The italicized abbreviations in parentheses indicate the physical state of each species. The common phases of matter are designated (s), (l), (g) for solid, liquid, and gas, respectively.
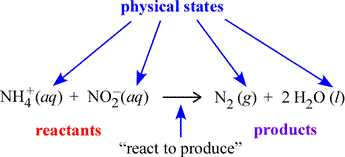
The (aq) seen at left designates a species dissolved in water to form what is called an aqueous solution. How we can translate, or read out, a chemical equation? Here, we could read the example equation as "an ammonium ion and a nitrite ion (in aqueous solution) react to produce one molecule of gaseous diatomic nitrogen and two molecules of water".
The above equation is balanced in that there are the same number of atoms of each type on the reactant and product sides, as required by the law of conservation of mass, which applies to chemical reactions. The act of balancing chemical equations - when initially in an unbalanced or skeletal form - is treated below.
The term "process" is more general than "chemical change", and we commonly use the symbolism of chemical equations to represent a variety of processes, e.g. physical changes:
H2O(l) → H2O(g)
We will in succeeding lectures, modify the basic chemical equation or add other features to indicate properties of the process it represents, such as energy changes and dynamic equilibrium. For now, bear in mind that the chemical equation is a symbolic language in chemistry that you ought to practice using at every opportunity. Mastering the symbolism is much like developing foreign language skills. One would not feel at home or be able to effectively interact with the society in a country speaking a different language without some fluency in that local language; so too understanding and using chemical equations will help you feel more at home in the world of chemistry. Many problems in chemistry are most clearly analyzed by writing the applicable chemical equations, and failure to do so often results in being led astray.
Balancing chemical equations is a skill best learned by practice. A redeeming feature of this type of problem is that carefully checking that your equation satisfies a simple criterion, you know whether or not you have the right answer. We refer to an unbalanced chemical equation as a skeletal equation. The skeletal equation is only a qualitative expression - it tells us what the reactants and products of a reaction are. It does not provide us with the quantitative, or stoichiometric relationship of the reactants and products. A balanced chemical equation satisfies the criterion that the number of atoms of each type of element is the same on both sides of the equation. That this must be true for a properly balanced chemical equation is a result of the law of conservation of mass.
Many chemical equations in their skeletal form can be easily balanced by inspection because of their relative simplicity. However, for more complex equations, a more systematic approach is advisable. The first principle of such an approach is to balance each atom type individually, and look at the equation to see how many formulas each atom type occurs in. One should start by balancing the atom type(s) that occur in the fewest number of formulas, and leave the atom type that occurs in the greatest number of formulas for last. Another principle to use to to recognize groupings of atoms in the formulas - such as polyatomic ions - that remain unchanged in the reaction, and treat them the same as if they were atoms.
Examples of balancing chemical equations
Example 1: Complete combustion of octane (C8H18).
Example 2: A second example.
Example 3: Balance the following skeletal chemical equation:
ClO2 + H2O → HClO3 + HCl
Solution: To balance any equation, we need to keep track of the numbers of atoms on each side, trying to use coefficients for the atoms and molecules involved so the numbers of each atom type are the same on both sides. This is an iterative bookkeeping process, and sometimes it may seem that we just have to try things until something works. First off, let's see where we stand with the reaction as it is written:
| atom | reactants | products | ClO2 + H2O → HClO3 + HCl Things look bright - there are three types of atoms to balance, and two of them are already balanced. But Cl is not balanced, and there is only one choice at this point: We must multiply the ClO2 species on the reactant side by 2 to bring the Cl atoms into balance. But this changes the number of O atoms. So we have: |
|||
| Cl | 1 | 2 | ||||
| H | 2 | 2 | ||||
| O | 3 | 3 | ||||
| atom | reactants | products | 2 ClO2 + H2O → HClO3 + HCl Now H and Cl are both balanced, but we have a problem with O. But note that if we multiply the O-containing species on the reactant side by 3, and the O-containing species on the product side by 5, we should get the same number of O atoms on each side. We keep our fingers crossed in hopes that this doesn't throw anything else out of balance: |
|||
| Cl | 2 | 2 | ||||
| H | 2 | 2 | ||||
| O | 5 | 3 | ||||
| atom | reactants | products | 6 ClO2 + 3 H2O → 5 HClO3 + HCl It worked! Our equation is balanced. It must always be possible to balance an equation representing any legitimate chemical reaction. |
|||
| Cl | 6 | 6 | ||||
| H | 6 | 6 | ||||
| O | 15 | 15 | ||||