Lecture 23. Biological oxidation-reduction reactions
Thursday 18 April 2019
Oxidation-reduction ("redox") reactions 101. Biological redox reactions. Assignment of oxidation states to carbon atoms. Writing half-reactions, and combining them into a complete redox reaction. Reduction potentials and standard reduction potentials. Using biochemical reduction potentials to calculate free energy changes for biochemical redox reactions. Nicotinamide and flavin nucleotides as modular substrates in biochemical redox reactions.
Reading: Lehninger - Ch.13, pp.517-527.
Summary
Reading summary. §13.4 Biological oxidation-reduction reactions. The flow of electrons can do biological work. Oxidation-reduction reactions can be described as half-reactions. Biological oxidations often involve dehydrogenation. Reduction potentials measure affinity for electrons. Standard reduction potentials can be used to calculate free energy change. Worked example 13-3: Calculation of ΔG°′ and ΔG of a redox reaction. Cellular oxidation of glucose to carbon dioxide requires specialized electron carriers. A few types of coenzymes and proteins serve as universal electron carriers. NADH and NADPH act with dehydrogenases as soluble electron carriers. NAD has important functions in addition to electron transfer. Dietary deficiency of niacin, the vitamin form of NAD and NADP, causes pellagra. Flavin nucleotides are tightly bound in flavoproteins.
Electrons accepted by biological electron carriers (NADH, FADH2) at a relatively low reduction potential are in effect donated - via the electron transport chain - to oxygen, which is at a high reduction potential.
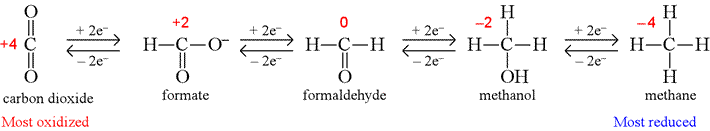
Oxidation numbers and biologically-relevant compounds.
Two examples of oxidation states for compounds of biological relevance are those for the functional groups in the redox reactions of energy metabolism, and reactive oxygen species (ROS), agents damaging to cells that are also linked to aging.
The following shows how the oxidation number of the carbon atom changes for the series of one-carbon molecules containing C, H, and O only.
Related topics pages: