BIOCHEMISTRY TOPICS
Collagen
Collagen amino acid composition and sequence. The collagen triple helix.
Collagen refers to a group of fibrous proteins found mainly in connective tissue. Collagen consists of extensively crosslinked molecules of tropocollagen, which is a trimeric protein of 285 kD. The polypeptide chains of tropocollagen coil around each other in a triple helix conformation.
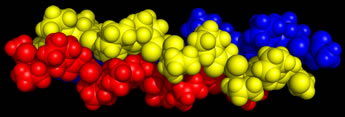
Left: Space-filling model of a short section of collagen triple helix. Each polypeptide chain is a different color.
These polypeptides have an amino acid sequence that consists largely of the three-fold repeat Gly-X-Y, where Gly is glycine and X and/or Y is often proline. In many cases, proline at Y (preceding Gly) is often hydroxylated post-translationally, converting it to 4-hydroxyproline (Hyp). Lysine residues at Y are also similarly hydroxylated in some cases to 5-hydroxylysine. Another modification leads to 3-hydroxyproline (Hyp) residues. Hydroxylation is a process dependent on ascorbate (vitamin C), which is why the disease scurvy displays symptoms due to defective collagen. The details of the structure of the tropocollagen triple helix show that the individual chains adopt a left-handed helical conformation similar to that shown by poly-Pro type II and poly-Gly type II, which are model synthetic homopolymers. Note the regularity of the collagen sequence, in which the tripeptide Gly-Pro-Hyp is particularly prominent. A regularity in sequence is a characteristic feature of structural (fibrous) proteins, or structural domains, and collagen is a particularly striking case. Globular proteins generally display relatively little sequence regularity, though a great many functionally-relevant sequence motifs have been described.
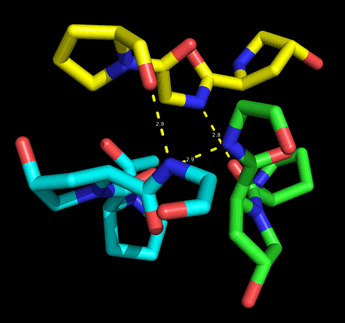
The collagen triple helix viewed along its superhelical axis shows that the proline and hydroxyproline residues are on the periphery of the cylindrical envelope of the triple helix, while the glycine residues approach the axis. The small hydrogen R group of glycine is critical for this arrangement, and for the regular structure of collagen. Thus, it is necessary for every third residue in collagen to be Gly. The amide nitrogen of each Gly donates an interchain hydrogen bond to the acyl oxygen of residue X. The image at right shows a fragment of each of the three chains of a model for the collagen triple helix that illustrates how each layer (three-residue repeat) forms by the intertwining of the chains in this axial view.
Higher-order structures
Crosslinking between chains occurs due to post-translational modification of Lys residues to form allysine which subsequently react with residues on adjacent chains. Allysine, histidine, and hydroxylysine residues participate.
Related topics pages: