BIOCHEMISTRY TOPICS
Secondary structure
Types of secondary structure. Summary of regular polypeptide chain conformations.
Linderstrom-Lang first described protein structure in terms of a hierarchy, in which the covalent linkage of the polypeptide chain is referred to as its primary structure. The next level is secondary structure - the adoption (by segments of the main chain) of regular conformations exhibited by the α helix (alpha helix) and the β strand that makes up β (Beta) sheets. Together with turns and loops, these two types of secondary structure can be assembled into a three-dimensional arrangement that constitutes the next level of the structural hierarchy, tertiary structure. Ultimately, of great significance to tertiary protein structure is the fact that secondary structure provides a means to pair the polar groups of the main chain in hydrogen bond interactions. Whatever the local chain conformation may be in each part of a tertiary structure, the main chain must traverse or occupy some of the volume of the nonpolar interior of a protein. Unpaired polar groups in nonpolar environments are notably unfavorable energetically. Hence, in participating in secondary structure formation, portions of polypeptide chain within proteins avoid unpaired polar main chain groups, contributing to stability of the folded conformation.
Of noncovalent forces, hydrogen bonding is most crucial to these secondary structural elements. For an α helix, the acyl oxygen atom at residue n accepts a hydrogen bond from the amide group of residue n + 4. The β sheet structure is stabilized by hydrogen bonds between carbonyl and amide groups of adjacent strands.
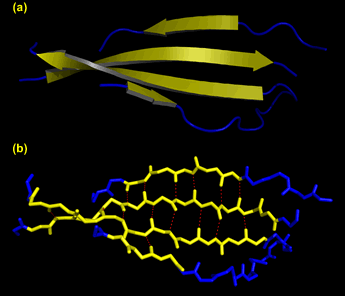
Left: An antiparallel β sheet. (a) Ribbon representation, with β strands as yellow arrows and connecting segments as blue wires. (b) Same view as in (a), but showing the main chain backbone. The red dashes show the hydrogen bond interactions between strands in the sheet. The distances between the heteroatoms at either end of the dashes ranges from 2.6 to 3.3 Å. These images were generated from pdb file 1fcc, and show only a portion of the structure of an antibody.
Summarizing regular polypeptide chain conformations
Regular polypeptide conformations such as an α helix and the β strands that make up antiparallel and parallel β sheets can be characterized by a set of geometric parameters that correspond to "ideal" values. Bear in mind that in actual protein structures, there can be considerable variation around these parameters since the conformational energies are dependent on sequence.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Related topics pages:
- circular dichroism (CD) spectroscopy
- α helix
- β strand
- tertiary protein structure