BIOCHEMISTRY TOPICS
Protein methods
Separation and purification of proteins. Chromatography. Electrophoresis. Measuring protein amounts and activity.
Protein purification and analysis
In order to study a protein, it is typically necessary from an experimental point of view to produce a sample of the protein in a reasonably pure form. Accordingly, the laboratory methods for protein purification merit discussion. It is relevant and helpful to distinguish between preparative and analytical methods of fractionation that are used to produce samples that are enriched or purified with respect to a given target protein. This distinction is a matter of scale, with preparative methods, principally chromatography, yielding a larger scale sample of a protein useful for further characterization or study. We'll consider large scale to be on the order of milligram (mg) quantities or more. For example, an enzyme sample produced on a preparative scale is used for kinetic and inhibition studies, or a given protein is produced in quantity for crystallization trials that are a necessary prerequisite for determination of its tertiary structure by the method of X-ray crystallography. On the other hand, analytical methods, such as gel electrophoresis and capillary electrophoresis yield very small quantities. Analytical-scale methods are useful as means of assessing the progress of a preparative-scale method of purification, or as the starting point for further analysis that can be performed using minute quantities of material, such as picomole-scale peptide sequencing by Edman degradation, or mass spectrometry measurements. The increasing technological sophistication of methods used for the characterization of protein samples, particularly their sensitivity, has made it possible to obtain a wealth of information from analytical-scale preparations.
Preparative methods: Salting in/salting out; Chromatography (ion exchange, hydrophobic interaction, gel filtration, affinity).
Purifying a protein requires a strategy.
Salting out separates proteins by their solubility.
Chromatography involves interaction with mobile and stationary phases
Electrophoresis separates molecules according to charge and size
Both ion exchange chromatography and isoelectric focusing depend on the charge of the species being fractionated. Here, we review the principle of the variation of charge of polyelectrolyte species such as a polypeptide chains with the pH of their surrounding environment.
- Definition: The pI, or isoelectric point, of a polyelectrolyte is the pH at which the net charge on the molecule is zero.
- If pH > pI, then the molecule is negatively charged (e.g. acidic proteins have pI < 7)
- If pH < pI, the molecule will be positively charged (e.g. basic proteins have pI > 7)
The second two items on the list are a kind of broad-brush principle species analysis applied to a multiprotic molecule. The molecule at the isoelectric point is analogous to the zwitterionic intermediate form of a diprotic amino acid. The positively-charged molecule at pH < pI is similarly analogous to the fully protonated (and positively-charged) amino acid, and finally the negatively-charged molecule at pH > pI is analogous to a fully deprotonated (and negatively-charged) amino acid. In fact, however, we can say more, for the further away the pH is from the isoelectric point of the protein, the more charge it carries (positive or negative, depending on whether we are below or above the pI).
The figure below illustrates these ideas on how the charge on a protein would vary with the "ambient" pH of the medium of which it is a component.
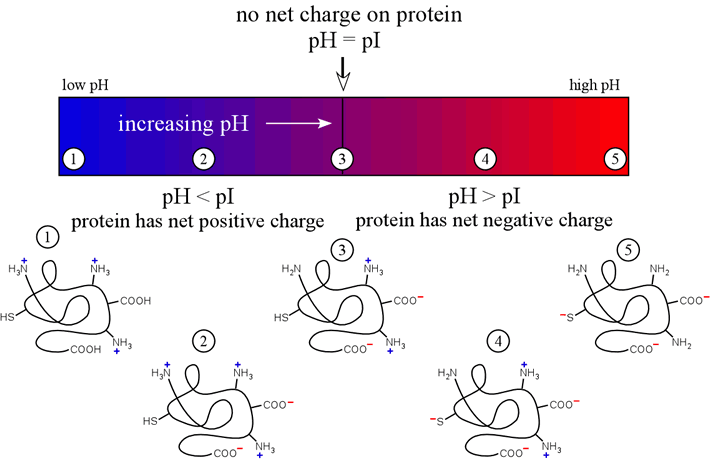
The above diagram represents a polypeptide chain with a pI value near neutrality. We ought to also consider how polypeptide pI varies generally with amino acid composition.
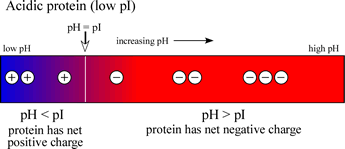
If a protein contains a high content of acidic residues, these being aspartate and glutamate, and a relatively low content of basic residues (lysine, arginine), then the pI value will be in the acidic range (<7). The charge vs. pH diagram for such an acidic protein is shown at left.
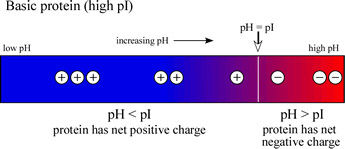
If, on the other hand, a protein contains a high content of basic residues and a relatively low content of acidic residues, then the pI value will be in the basic range (>7), and the diagram at right will be representative of such a case.
Note that an acidic (low pI) protein will carry a net negative charge at a neutral pH, while a basic (high pI) protein will bear a net positive charge at a neutral pH.
Related topics pages:
- Circular dichroism (CD) spectroscopy
- NMR: Summary of multidimensional NMR as a method of protein structure determination
- Acid-base chemistry: Polyprotic systems