BIOCHEMISTRY TOPICS
Circular dichroism (CD) spectroscopy
CD spectroscopy - a synopsis of the technique. Using CD to measure the stability of a protein.
Synopsis of the technique: Circular dichroism (CD) spectroscopy is mainly used as a method for measuring the secondary structure content of a protein. It is typically used as a way of monitoring the unfolding transition (thermal or chemical denaturation). Since an unfolded protein is deviod of secondary structure, monitoring the CD signal at, say, 222 nm enables an experimenter to follow the progress of unfolding as a function of temperature or concentration of denaturant. The CD spectrum of an unfolded protein should resemble the random coil spectrum shown in the figure.
Dissymmetric (or chiral) molecules will rotate the plane of plane-polarized light. They also show differences in absoerption of left vs. right circularly polarized light.
Sources of dissymmetry in proteins:
- Configurational: chiral or asymmetric carbon atoms (e.g. Cα in all non-Gly amino acids)
- Conformational: secondary structure features (e.g.α helices are right-handed)
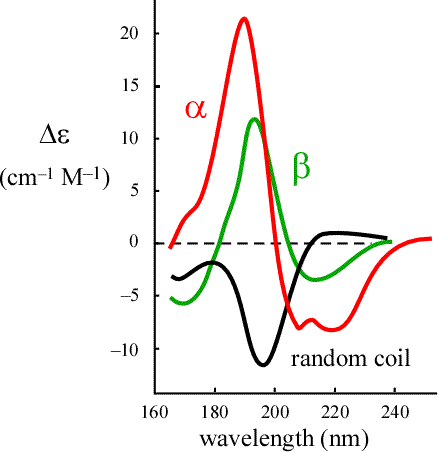
A CD spectrum is obtained by measuring the diffenence in absoerption of left- and right-circularly polarized light by a solution of a macromolecule. The far-UV spectrum (170-240 nm) gives an estimate of the secondary structure content of a protein.
Using CD to measure stability of a protein
The van't Hoff relation (below) can be used for the non-calorimetric determination of thermodynamic parameters of enthalpy, entropy, and the free energy. The enthalpy (H) (2) can be determined from the temperature variation of the equilibrium constant (Keq). The loss of the native conformation (3) by an increase in temperature or pressure, or by the addition of some denaturing agent, has been shown to be completely reversible for a number of proteins.
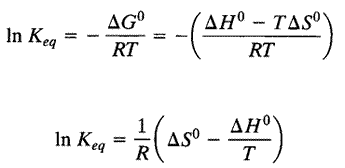
The van't Hoff relation. If we re-write the equation relating ΔG° to lnK and substitute in the definition of ΔG° in terms of ΔH° and ΔS° at constant temperature and pressure, we obtain the equation at left. Further analysis shows that lnK is a linear function of 1/T, with slope −ΔH°/ R.
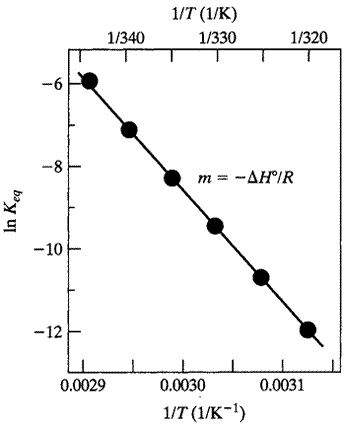
At right is shown a van't Hoff plot of lnK vs 1/T.
If the K in question is the equilibrium constant for folding, the assumption of a two-state, reversible transition implies that the measured enthalpy of transition should correspond to the van't Hoff enthalpy.
Related topics pages: