BIOCHEMISTRY TOPICS
Quaternary structure
Quaternary structure arises from associations between separate polypeptide chains. Symmetry in quaternary structure. Example quaternary structures.
Quaternary structure is highest level of the hierarchical description of protein structure, applying to proteins composed of more than one polypeptide chain. In this case, the individual chains are called subunits, and the quaternary structure of the protein is the manner in which subunits assemble to form the complete complex or holoprotein. Associations of identical (or very similar) subunits (monomers) in a symmetric arrangement is a predominant feature of quaternary structure. Two subunits constitute a dimer, three a trimer, four a tetramer, and so on. Quaternary structure can give rise to more complex functional properties in proteins, as compared to their monomeric counterparts. The classic example of this is provided by the oxygen-binding proteins, myoglobin (monomer) and hemoglobin (tetramer). The latter shows oxygen binding properties characteristic of an intersubunit cross-talk called cooperativity.
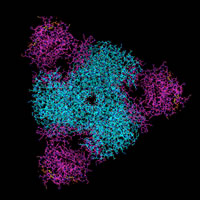
ATCase - aspartate transcarbamoylase, an enzyme at the beginning of the pathway for pyramidine synthesis, provides a striking example of quaternary structure. ATCase forms a heterododecamer (12 subunits) constructed of two catalytic trimers separated along a 3-fold rotational symmetry axis bridged by three regulatory dimers. The view at left is along the 3-fold symmetry axis, with the catalytic chains shown in cyan, regulatory chains in magenta.
Related topics pages: