Lecture 25. Biochemical signaling
Monday 7 November 2016
Hormones. Pancreatic peptide hormones, steroids. Heterotrimeric G proteins. Adenylate cyclase, cyclic AMP, and protein kinase A.
Reading: VVP4e - Ch.13, pp.396-402, 417-426.
Summary
Hormones
Hormones are molecules that act as messengers in a living organism, causing specific responses in target cells or tissues. Typically, hormones are polypeptides, steroids, or derivatives of amino acids. The effects and actions of hormones can be long-range and fairly long-lived - such is the case for endocrine hormones such as epinephrine, glucagon, and insulin. Paracrine hormones have a more localized action, and autocrine hormones are those whose action is limited to the cells that produced them.
G proteins
G proteins are a large class of signaling proteins that are activated by binding guanosine triphosphate (GTP), and are deactivated as a result of an intrinsic GTPase activity. There are actually two classes of G proteins: heterotrimeric G proteins, consisting of α, β, and γ (alpha, beta, and gamma) subunits that are coupled to receptor proteins that are integral membrane proteins with the architecture of seven transmembrane α helices - these are commonly denoted G protein coupled receptors (GPCRs); and small G proteins, monomeric members of the Ras superfamily.
As a paradigm of the signal transduction mediated by heterotrimeric G proteins coupled to GPCRs, the case of the pancreatic hormone glucagon binding to glucacon receptors exposed on the surface of hepatocytes (liver cells) serves well. When glucagon binds to the GPCR glucagon receptor, it causes a conformational change in the latter that affects its interaction with the Gα subunit of its associated heterotrimeric G protein, in turn inducing the Gα subunit to exchange its bound GDP molecule for GTP. This activation entails the dissociation of the Gα subunit from the Gβγ subunit; these dissociated parts of the hetreotrimer have separate downstream signaling effects. The Gα subunit, in this case denoted Gsα, stimulates the activity of another membrane-associated protein, adenylate cyclase. This results in the intracellular production of 3′,5′-cyclic AMP (cAMP), a so-called "second messenger" that signals further downstream effects such as a protein kinase cascade that stimuates the liver to produce more glucose for export to the bloodstream by turning on within hepatocytes the activity of glycogen phosphorylase via its phosphorylation
Protein kinase A (PKA)
A kinase is an enzyme that transfers a phosphate group - usually from the γ-phosphate from ATP - to a substrate molecule. If the substrate is a protein, the enzyme is referred to as a protein kinase. Kinases are ubiquitous metabolic and regulatory enzymes, and mediate links of signal transduction pathways. A particularly important and instructive example of the latter is protein kinase A (PKA, or cAMP-dependent protein kinase, cAPK) Once specifically activated by cAMP, PKA [EC 2.7.11.11] catalyzes the phosphorylation of a variety of intracellular targets, including the bifunctional phosphofructokinase/fructose 2,6-bisphosphatase, phosphorylase kinase, glycogen synthase, hormone-sensitive lipase, and acetyl CoA carboxylase.
The kinase is a heterotetramer of two catalytic and two regulatory subunits. The binding of cAMP to regulatory subunits triggers the dissociation of the complex, liberating free catalytic subunits.
The reaction the catalytic subunit of PKA carries out is
ATP + protein = ADP + phosphoprotein
Protein kinase A is a key component of a signal transduction pathway that is mediated by cAMP. The latter is often termed a second messenger, in that it acts intracellularly to transmit an extracellular signal (such as the binding of a hormone to a cell surface receptor). A principal result of the activation of PKA, via intracellular production of cAMP, is the breakdown of glycogen in the liver and subsequent transport of glucose to the bloodstream, maintaining blood glucose levels between meals.
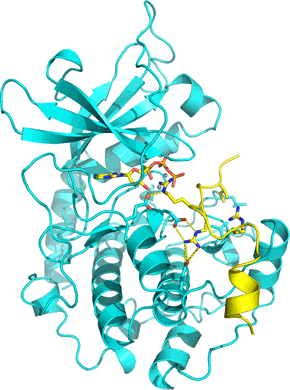
Right: The structure of the catalytic subunit of PKA (PDB: 1atp) shown as a cyan ribbon. The subunit has a two-lobed structure with a cleft between them where substrate ATP (shown in stick form) binds. A 20-residue peptide that includes a pseudosubstrate sequence is also bound in the active site cleft (shown in yellow), forming a ternary complex. The pseudosubstrate peptide forms part of the regulatory subunit of PKA holoenzyme.