BIOCHEMISTRY TOPICS
Thiamine pyrophosphate (TPP)
Decarboxylation reactions. Decarboxylation of α-keto acids. Structure and properties of TPP. Role of TPP in non-oxidative and oxidative α-keto acid-decarboxylation.
Thiamine pyrophosphate (TPP, or thiamine diphosphate, TDP) is the active form of the vitamin thiamine. TPP is an important cofactor that acts catalytically in the decarboxylation of α-keto acids and the transketolase reaction. In the mechanism of TPP-dependent enzymes, the cofactor is a carrier of hydroxyalkyl residues (also referred to as "active aldehydes")
Decarboxylation of α-keto acids
Decarboxylation of an α-keto (2-oxo) acid presents a serious mechanistic challenge, as it seems to require the generation of a carbanion on a carbonyl, an intermediate not observed in reactions of carbonyl compounds.

The α-keto acid decarboxylations that occur in biochemistry can be non-oxidative or oxidative, as illustrated at left for pyruvate The non-oxidative decarboxylation of pyruvate yields acetaldehyde plus carbon dioxide, while the formal product of its oxidative decarboxylation is acetate and CO2.
A decarboxylation of a carboxylic acid in general requires an electron sink of some kind. In β-keto acid decarboxylation, the keto group is appropriately located to stabilize, via resonance, the carbanion that formally arises.
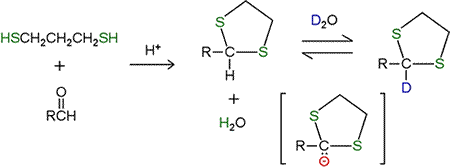
The keto group of an α-keto acid cannot achieve such stabilization upon decarboxylation, so the trick would be to extend, or convert the carbonyl group into something that can act as an electron sink. In organic chemistry, a dithiol derivative of an aldehyde or ketone is employed for some purposes. The reaction of 1,3-propanedithiol with an aldehyde produces a cyclic thioacetal. This product in the presence of D2O solvent shows hydrogen exchange, presumably via the carbanion intermediate shown. The presence of the neighboring sulfur atoms stabilizes the negative charge of this intermediate.
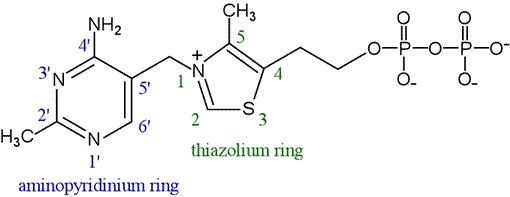
Thiamine pyrophosphate, the full structure of which is shown at right, is analogous to the dithiol derivative of an aldehyde, the thioacetal shown above. How does it work? The most informative clue to the mode of action of TPP came from the observation in an NMR experiment that the proton attached to C2 of the thiazolium ring also exchanges with deuterium in D2O solvent. The proton exchange observation suggested the chemical property of TPP that underlies its co-catalytic activities is its ability to stabilize the carbanion formed by loss of H+ from carbon 2 (on the thiazolium ring). This stabilization is achieved through the adjacent sulfur and positively-charged nitrogen atoms.
The carbanion then acts as a strong nucleophile toward
the carbonyl carbon of a substrate molecule, forming a tetrahedral
adduct. The decarboxylation is then facilitated by the ability of
the TPP part of the addition compound to act as an electron sink,
again stabilizing a carbanion intermediate, this time via resonance.
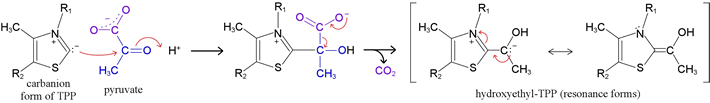
The species formed by decarboxylation of pyruvate, hydroxyethyl-TPP (shown above, at right), is of utility as a biosynthetic agent by virtue of its ability to transfer the two-carbon fragment to a variety of electrophilic carbon atoms (more below).
Reactions assisted by TPP
Enzymes carrying out decarboxylation and transketolation reactions, such as the pyruvate dehydrogenase complex (PDH complex) and transketolase [EC 2.2.1.1], rely on TPP as a catalytic cofactor.
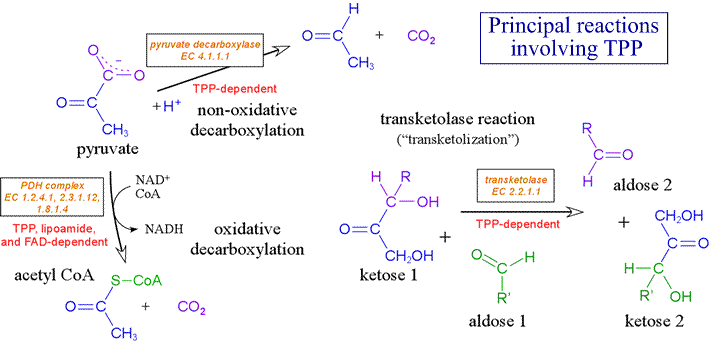
In the PDH complex, TPP plays an essential role in the of the 2-oxoacid (α-keto acid) pyruvate. Its role is identical in the α-ketoglutarate dehydrogenase complex, which converts 2-oxoglutarate (α-ketoglutarate) to succinyl CoA. This conversion occurs within the citric acid cycle. In humans and other animals, vitamin B1 or thiamine, a required dietary component, is the precursor of TPP.
Hydroxyethyl-TPP
Hydroxyethyl-TPP, derived from the TPP-assisted decarboxylation of pyruvate, is invoked for biosynthesis - for example, in the amino acids leucine, isoleucine, and valine. The addition of hydroxyethyl-TPP to a second pyruvate molecule leads to valine and leucine, while addition to α-ketobutyrate leads to isoleucine.