BIOCHEMISTRY TOPICS
β strands and β sheets
The β strand conformation and the alignment of β strands to form β sheets. The types of β sheets and the β hairpin motif.
One of the predominant secondary structure features of proteins, β sheets (beta sheets) are formed by the juxtaposition of sections of polypeptide chains in relatively extended conformations known as β-strands. While β sheets come in two basic flavors, parallel and antiparallel, according to the orientation of the component strands. β-sheets in which some adjacent strands are parallel, while others are antiparallel, termed mixed β-sheets, are less frequently observed. The β-sheet structure is stablized by hydrogen bonds between carbonyl and amide groups of adjacent strands, while the side chains of the residues project above and below the plane of the sheet. The β-sheets observed in proteins all show varying degrees of twist - that is, the sheets are rarely, if ever, perfectly "flat".
In contrast to an α helix, the polypeptide chain in a β-strand is
closer to being fully extended, which would correspond to
φ
= ψ = 180°. In this sense, the
β-strand is a regularly
repeating main chain conformation, just like the α-helix. A fully extended (φ
= ψ = 180°) polypeptide can be thought of as a helix with a 180°/residue
twist (2 residues/turn) and a translation per residue of 3.5 Å
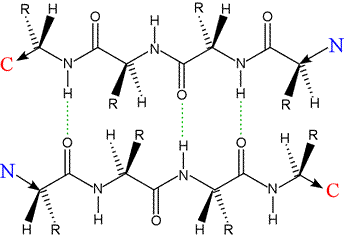
Left: Two polypeptides in extendend conformation aligned in an antiparallel orientation. Hydrogen bonds are indicated by dashed green lines. The extended polypeptides form β-strands, and their interaction shown here forms the architectural basis for an antiparallel β sheet. The residues of each strand are in register and the the R groups of the proto-sheet alternate in projecting above and below a planar surface defined by the sheet.
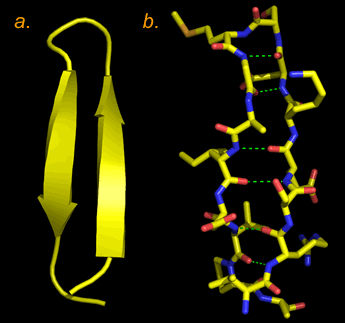
Right: Two representations of a β hairpin structure, two antiparallel β strands connected by a reverse turn. In panel a, the structure is shown abstractly, with the β strands shown as ribbons (the arrow points in the direction along the polypeptide chain from the N terminus toward the C terminus), and other parts of the polypeptide main chain as a wire. In panel b, the non-hydrogen atoms are all shown in a "sticks" format (atoms are located at vertices and at ends of sticks, C in yellow, N in blue, O in red, and S in orange. Hydrogen bonds between main-chain amide and carbonyl groups are shown as dashed green lines. The characteristic twist of the β strands is evident in both images. The structure is that of the first 18 residues in an X-ray crystallographic structure for the enzyme fumarase. (PDB: 1fuo).
The reiterated alignment of adjacent β-strands in a parallel fashion leads to the formation of a parallel β sheet.
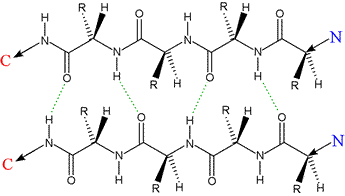
Left: Two polypeptides in extendend conformation aligned in a parallel orientation, the architectural basis for a parallel β sheet. Hydrogen bonds, indicated by dashed green lines, show a less ideal geometry compared to the antiparallel case, with a given residue making H-bonds to residues in the adjacent strand out of register by ±1. Like the case of the antiparallel strands, however, the R groups of the proto-sheet alternate in projecting above and below the plane of the sheet.
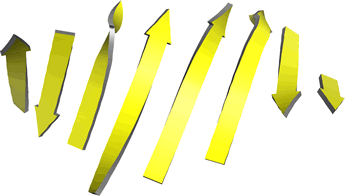
Left: Ribbon diagram of β-strands from an eight-stranded mixed β-sheet from carboxypeptidase A. The order of the strands (by sequence) is, from left to right, 1 2 4 3 5 8 6 7. Note the twist in each β-strand as well as the twist in the sheet (viewed along a horizontal line in the middle of the plane of the figure).