|
|
|
|
|
|
|
|
|
|
|
Special topic: Arachidonate,
Eicosanoids, and Endocannabinoids |
|
|
|
|
|
|
|
|
|
|
|
|
[ Wed 02-27-02 ] |
|
|
|
CHEM 445:
[ home ] [ syllabus
] |
|
|
|
|
|
|
|
|
|
|
|
|
Reading: Stryer, Ch.24, pp.624-625 |
|
|
[ Back to Lecture
14 ] |
|
|
|
|
|
|
|
|
|
|
|
|
This page was last updated on 27-Feb-2002 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Synopsis: Arachidonate is derived from
the essential fatty acid linoleate. Linoleic acid is an w-6
fatty acid, and the dietary requirement for w-6
fatty acids is at least in part due to its conversion to arachidonate, which
is itself a precursor of several important classes of signaling molecules.
These molecules, collectively referred to as eicosanoids, are involved in
a large variety of physiological processes, such as inflammation and other
aspects of immune system response, regulation of blood flow and clotting,
ion transport, synaptic transmission, and reproductive phenomena. The major
classes of eicosanoids are the prostaglandins, prostacyclins, thromboxanes,
and leukotrienes. In addition, neutral derivatives of arachidonate, such
as anandamide (arachidonylethanolamide) and 2-AG (sn-2-arachidonylglycerol)
have been found to serve as natural ligands for cannabinoid (CB) receptors.
The pharmacological effects of these endocannabinoids suggest that they
participate in regulating mood, memory, appetite, and perception of pain.
CB receptor agonists have been reported to have a role in control of neuronal
excitability. Thus, consideration of the metabolic provenance and fate of
arachidonate leads into a complex, yet fascinating story that weaves together
aspects of biochemistry, nutrition, immunology, neurobiology and pharmacology.
The unfolding of this story is at a relatively early stage, and investigations
relating to arachidonate derivatives are current and active areas of research. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
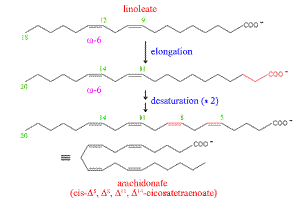
Arachidonate is derived from linoleate
by elongation and desaturation |
|
|
|
|
|
|
|
 |
|
|
|
|
|
Given the availability of linoleate from dietary sources,
the synthesis of arachidonate can proceed by means of the elongase
and desaturase activities associated with the smooth ER. The scheme
at left shows one possible pathway. |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
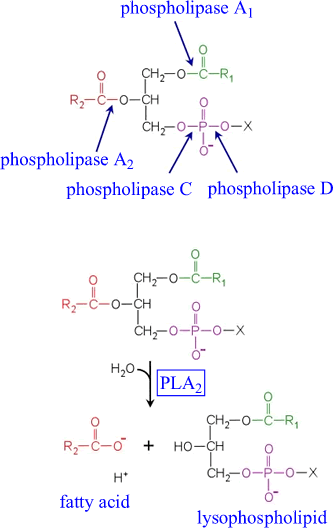
Arachidonate is stored as an ester
in membrane phospholipids |
|
|
|
|
|
|
|
Very little free arachidonate exists in
cells. Instead, it is typically stored as an ester in membrane phospholipids.
The fatty acids in membrane phospholipids are mobilized by phospholipase
enzymes. Phospholipases are classified according to which bond of
the phospholipid is hydrolyzed, as shown in the figure. |
|
|
|
|
|
|
|
 |
|
Analysis of membrane phospholipids has shown that saturated
fatty acids predominate at the sn-1 position of 3-phosphoglycerol
derivatives, while unsaturated fatty acids predominate at the 2 position.
Thus, arachidonate is typically liberated by phospholipase A2
(PLA2). Alternatively, arachidonate may be modified within
the membrane itself, and PLA2 action would then liberate
the arachidonate derivative. Either way, it provides an example of
how membrane phospholipids are themselves integral parts of signaling
pathways of cells. Another example is that of phosphoinositol phosphates
(X in the figure at left would correspond to a phosphorylated form
of inositol). The activity of PLA2 could itself be regulated
by some type of signal, perhaps a rise in intracellular [Ca2+],
leading to the release of arachidonate, and its subsequent conversion
to the signaling molecules derived from it, as explained further below. |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
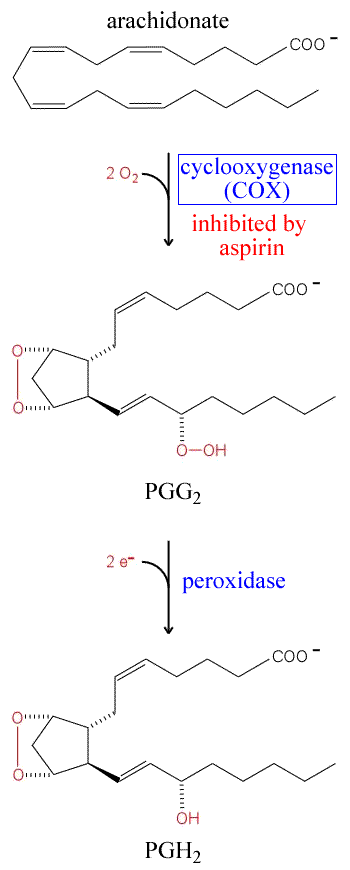
Cyclooxygenase enzymes convert arachidonate
into eicosanoid signaling molecules |
|
|
|
|
|
|
|
Much of the arachidonate that is destined to become
eicosanoid molecules is converted to the common precursor of prostaglandins,
prostacyclins, and thromboxanes by PGH2 synthase - also
sometimes referred to as prostaglandin synthase, but quite commonly
referred to simply as cyclooxygenase (COX). The synthase has two
distinct activities - a cyclooxygenase activity that utilizes molecular
oxygen to attach peroxide to arachidonate, while at the same time
introducing a cyclopentane ring. This intermediate product is referred
to as PGG2. A subsequent reductive step - the peroxidase
step - takes place at distinct site within the enzyme, and forms
the product PGH2. This is itself quite labile, and is
normally converted into one of many eicosanoid derivatives, depending
on the context. Isomerases convert PGH2 into the different
types of prostaglandins. Prostacyclin synthase converts PGH2
into PGI2, which undergoes further conversions. Thromboxane
synthase converts PGH2 into TXA2, which can
also undergo further conversion (see figure below).
An interesting pharmacological feature of COX enzymes is that they
are inactivated by aspirin (acetylsalicylate). The inactivation
is due to aspirin's ability to acetylate a serine residue of COX
that is necessary for catalysis. Although aspirin itself was one
of the first drugs ever synthesized, and herbal remedies based on
plants containing salicylate derivatives have been used for centuries,
the mode by which aspirin exerts its effects was not understood
until 1971, when John Vane discovered that aspirin blocked the formation
of prostaglandins and thromboxanes. The therapeutic utility of aspirin
is now thought to be largely due to this effect, as these eicosanoids
have an impressive range of physiological effects. Sune Bergström,
Bengt Samuelson, and Vane were awarded the 1982
Nobel Prize in Physiology or Medicine for their discoveries
concerning "prostaglandins and related biologically active
substances". |
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Coming soon:
More of the story....stay tiuned! |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
References
Campbell WB & Halushka PV. (1996) "Ch.26: Lipid-derived
autacoids" from Goodman & Gilman's The Pharamacological
Basis of Therapeutics (9th ed., McGraw-Hill)
Christie Mc & Vaughn C. (2001) "Cannabinoids act backwards"
(News and views). Nature 410: 527-530.
DiMarzo et al. (2001). "Leptin-regulated endocannabinoids
are involved in maintaining food intake" Nature 410:
822-825.
Kurumbail RG, Kiefer JR, Marnett LJ. (2001) "Cyclooxygenase
enzymes: catalysis and inhibition" Curr Opin Struct Biol
11: 752-760.
Mechoulam R & Fride E. (2001) "A hunger for cannabinoids"
(News and views). Nature 410: 763-765.
Wilson RL & Nicoll RA. (2001) "Endogenous cannabinoids
mediate retrograde signalling at hippocampal synapses" Nature
410: 588-592. |
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
| |
|
|
|
|
|
|
|
|
|


