GENERAL CHEMISTRY TOPICS
Lewis structures: Octet rule violations
Expanded octets. Deficient octets and coordinate covalent bonds. Odd electron species.
As a generalization, the octet rule is a simple and effective guide to writing correct chemical structures. For the period 2 elements C, N, O, and F it is most strictly valid. However, there are three types of cases for which the octet rule must be relaxed. For many molecules with larger atoms (in Period 3 and higher) it is impossible to limit electrons around these atoms to an octet and draw Lewis structures that fairly represent the actual structures. Expansion of the number of valence electrons around these larger atoms must be allowed. This case of expanded octets is the principal one of three that can be described. The other two cases occur when valence elections are insufficient or odd in number.
Expanded octets
Expanded octets, exhibited by molecules such as XeF4, PCl5, and SF6 encompasses much of the structural chemistry of the heavier elements, and give rise to the more exotic molecular shapes of the single-center atom structures under consideration in this part of the course.
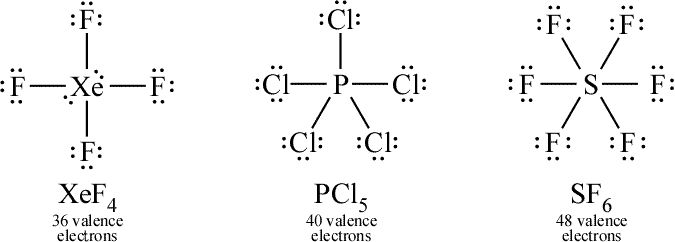
Above: Three examples of Lewis structures for which electrons surrounding the central atom must be expanded beyond an octet. Xenon tetrafluoride (XeF4), sulfur pentachloride (SF5), and phosphorous hexafluoride (SF6).
Incomplete octets
In some cases, when the procedure for drawing a Lewis structure is followed, the result is a central atom with less than an octet. Such incomplete octets arise for molecules with the Group 2A and 3A elements beryllium and boron, atoms with only a few valence electrons. For example, the Lewis structure for boron trihydride (BH3) cannot be drawn in a way that provides the central boron atom with a complete octet, since there are only six valence electrons total, and these are all used in the three covalent bonds formed. For boron trifluoride (BF3; 24 valence electrons), the analogous structure with an incomplete octet arises ((Lewis structure labeled (i), below). In this case, in principle one of the lone pairs of a terminal fluorine atom could be moved to form a double bond to complete an octet for boron (Lewis structure labeled (ii), below). However, this would result in the creation of formal charges. In particular, a positive formal charge borne by fluorine is considered unfavorable because of its high electronegativity. One might interpret this as a case in which two nonequivalent resonance structures can be drawn, with the structure bearing nonzero formal charges contributing very little to the resonance hybrid. In other words, we are led to conclude that the Lewis structure with an incomplete octet on the boron atom is the best, and the actual BF3 molecule “looks” very much like this.
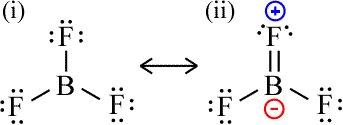
At first glance, this may appear to be a shortcoming in the symbolic representations of molecules using Lewis structures. However, the occurrence of an incomplete octet in the Lewis structure representation of a molecule signifies something valid and important about its reactivity. Since completing the octet of an atom lowers its energy, such molecules turn out to be reactive toward other molecules that can offer a lone pair to form a new covalent bond, called a coordinate covalent bond. The formation of the coordinate covalent bond completes the octet for such atoms.
Odd-electron and radical species
So far we have limited our Lewis structure examples to molecules and polyatomic ions with an even number of total valence electrons. This is in large measure due to the incompatibility of the octet rule with an odd number of electrons. But nature is not limited to even numbers! As in the case of incomplete octets, odd-electron species turn out to have an enhanced reactivity. As described in our text [Ref.1, p.228], molecules and ions with an odd number of electrons in their Lewis structure representations are termed radicals – or perhaps more often free radicals, a term that emphasizes both reactivity and mobility. Examples of such species are nitrogen monoxide (NO, also known as nitric oxide) and nitrogen dioxide (NO2).
So how do we draw Lewis structures for odd-electron species? Inevitably for an odd number of electrons, an atom will be left with an unpaired electron. Deciding which atom that is may pose a dilemma. We might consider minimizing formal charge. For example, with nitrogen monoxide (11 valence electrons), we can draw two structures.
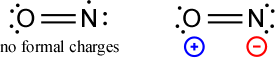
One of the structures places the single unpaired electron on the nitrogen atom, the other puts it on the oxygen atom. Assignment of formal charges shows that the former has no non-zero formal charge, while the latter has formal charges. Not only that, but the negative formal charge is carried by the less electronegative atom, nitrogen. For these reasons, we would judge the first structure, with the unpaired electron on nitrogen as better. If we considered the two structures as nonequivalent resonance forms, the species with formal charges would presumably contribute little to the resonance hybrid.
If we next consider nitrogen dioxide, NO2, with 17 valence electrons, the best Lewis structure is the set of equivalent resonance forms shown in the next figure, despite the fact that we can also draw two equivalent resonance forms in which the unpaired electron resides on an oxygen atom, and no atoms have a formal charge.

With these two examples, we might formulate the rule that the more electronegative atom is given a complete octet where possible, at least for atoms of period 2 elements which by our existing rules cannot expand beyond an octet. More to the point, we may ask what experimental evidence can be obtained for the presence of unpaired electrons in chemical species.
Although not all that commom, radicals are important because of their reactivity. The impact reactions involving free radicals can have is well demonstrated in biology and atmospheric chemistry.