GENERAL CHEMISTRY TOPICS
Electronegativity
Definition of electronegativity. Electronegativity scale for the elements. Bond dipoles.
The electronegativity (EN) of an atom is a measure of the degree to which it draws electrons to itself in context of a covalent chemical bond with another atom type. Thus, the electronegativity of an atom is closely related to the bond polarity of the bonds forming between that atom and other types of atoms. The polarity of a chemical bond places it in a continuum that in principle ranges from perfectly covalent (not polar at all, electrons equally shared between both atoms; e.g. H2, Cl2) to fully ionic (electron completely transferred from one atom to the other). Once a scale for electronegativity is specified, we can use the difference in electronegativity, ΔEN, between the pair of bonded atoms, as an estimate for bond polarity.
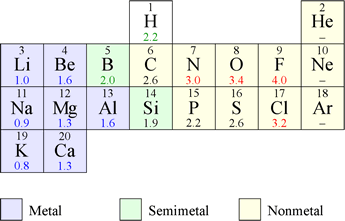
The electronegativities of elements 1 -20 are shown at left (values from Ref.1). Metals have the lowest electronegativities, nonmetals the highest. The general trend is for electronegativities to decrease as a group in the periodic table is descended, and increase from left to right along a period. Note that EN is not defined for the noble gas elements (group VIII) shown, as these do not form compounds.
The electronegativity of an element is not an intrinsic physical property that we can directly measure, such as molar mass, density, or melting point. One way to come up with a scale of electronegativity, however, is based on the measurable quantities electron affinity and ionization energy.
To illustrate the relationship between electronegativity and bond polarity, consider a hypothetical example.
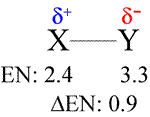
Suppose an atom of element X that has an electronegativity of 2.4 forms a bond with Y having EN = 3.3. The larger electronegativity of Y means the bonding pair of electrons are more likely to be nearer to the nucleus of Y than that of Z. This can be modeled as a small separation of charge along the X-Y bond axis. This charge separation, which is symbolized by the δ notation, creates a bond dipole. In this scheme, the δ's represent partial charges - that is, the charge δ is some fraction, between 0 and 1, of the magnitude of the charge of an electron.
Bond dipole moments
The dipole moment (μ) of a bond is a quantitative measure of bond polarity. The bond dipole moment is a vector quantity, having both magnitude and direction. Vectors are drawn as straight-line arrows, with the length of the line indicating the magnitude. An equation for the magnitude of the bond dipole moment shows that it is the product of the partial charge separation and the distance by which the charge is separated. This is taken to be the bond length.
μ = Q × r where Q = |δ| and r = bond length
Bond dipole moments are measured in units of "debye" (D) which is defined by the equivalence relation
1 D = 3.336 × 10−30 C·m
Bond dipole moments for bonds increase with increasing difference in electronegativity (ΔEN), which increases δ, and increasing bond length.