Lecture 19. Biological membranes
Thursday 4 April 2019
Structural, functional and dynamic properties of biological membranes. Integral membrane proteins.
Reading: Lehninger - Ch.11, pp.387-402.
Summary
Reading summary.
§11.1 The composition and architecture of membranes.
Each type of membrane has characteristic lipids and proteins.
All biological membranes share some fundamental properties.
A lipid bilayer is the basic structural element of membranes.
Three types of membrane proteins differ in the nature of their association with the membrane.
Many integral membrane proteins span the lipid bilayer.
Hydrophobic regions of integral proteins associate with membrane lipids.
The topology of an integral membrane protein can often be predicted from its sequence.
Covalently attached lipids anchor some membrane proteins.
Amphitropic proteins associate reversibly with the membrane.
§11.2 Membrane dynamics.
Acyl groups in the bilayer interior are ordered to varying degrees.
Transbilayer movement of lipids requires catalysis.
Lipids and proteins diffuse laterally in the bilayer.
Sphingolipids and cholesterol cluster together in membrane rafts (to p.402).
***
Biological membrane composition
See pp. 388-389
Membrane proteins
Clearly, the significant and sometimes quite high proportions of protein in biological membranes is indicative of functional importance. How can proteins become associated with or embedded in a lipid bilayer? From the perspecive of protein structure and stability, the polypeptide chain presents a problem. Organic solvents denature cytosolic proteins, suggesting that surface-exposed polar and charged groups in a nonpolar environment are energetically unstable. Hence the amino acid composition of a protein located within a biological membrane would be expected to present a greater proportion of amino acids with nonpolar side chains where its surface is exposed to the hydrocarbon fatty acid chains. However, the protein main chain is unavoidably polar. The best solution to the problem is for the polypeptide chain to adopt conformations that pair all polar groups in hydrogen bonds. The α helix accomplishes this beautifully, so if a single polypeptide chain is to traverse or embed in the lipid bilayer, an α helix with nonpolar side chains would seem to be a good option, certainly much preferable to an extended, β strand conformation.
The intergral membrane protein glycophorin, which spans the plasma membrane of erythrocytes illustrates the motif of a single transmembrane helix. Structurally, an integral membrane protein is one that has at least one segment of its chain traverse the membrane bilayer. Glycophorin A is as a protein of 131 residues, about 60 residues forming an extracellular N-terminal domain, while about 35 residues to the C terminus are intracellular. Bridging these is an α helix that traverses the membrane.
Further examples of integral membrane proteins illustrate multiple membrane-spanning secondary structure segments of the polypeptide chain. Bacteriorhodopsin is an integral membrane protein from Halobacterium salinarum, a halophilic archaeon.
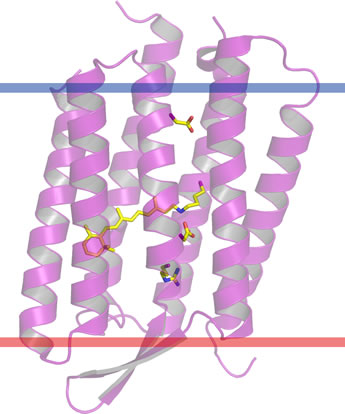
Its structure and approximate orientation in the prurple membrane patches that form in the plasma membrane is shown in the figure at left. The lower red bar is the extracellular membrane boundary, and the upper blue bar defines the intracellular side. The membrane-spanning portion of the structure consists of a bundle of seven α helices. This is in fact a common protein fold for integral membrane proteins, as the same seven transmembrane helix bundle is conserved in G-protein-coupled receptors (GPCRs). Bacteriorhodpsin is notable for its function as a light-driven proton pump, and as such is a central element of a bioenergetic paradigm. Its generation of a transmembrane difference in pH is analogous to the functioning of the much more complex electron transport chain in eukaryotic mitochondria. While in the latter, the input energy is provided by oxidation-reduction (redox) reactions, not light, similar principles appear to apply to the proton translocation mechanism.
Porins are a family of β-barrel (cylindrical β-sheet) integral membrane proteins that form channels through the plasma of gram negative bacteria. Porins are also found in the outer membranes of mitochondria and chloroplasts. As their name suggests, porins open relatively large pores, or channels in membranes, hence solution composition on either side of porin-containing membranes is free to approach equilibrium - at least with respect to all small molecules and ions.
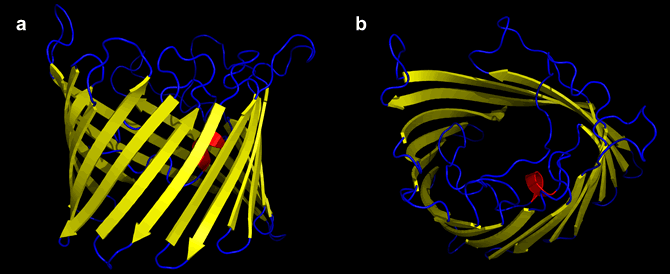
Above: Two views of the porin β barrel, (a) the view parallel to the membrane, (b) perpendicular to the plane of the membrane. Figure generated from pdb file 1PRN using PyMOL.
Lipid-linked proteins, and peripheral membrane proteins. (See pp. 395-397.)
Membrane structure and assembly
The fluid mosaic model, Molecular order and phases in lipid bilayers. Lateral diffusion, and FRAP experiments. Flippases, floppases, and scramblases. Membrane rafts. (see pp.397-402)
Related topics pages: