BIOCHEMISTRY TOPICS
Bacteriorhodopsin
Structure and function of bacteriorhodopsin. Absorption of light by the retinal prosthetic group in its ground-state trans configuration, results in its isomerization to the cis configuration, followed by its stepwise exergonic reversion to the ground state. Conformational changes accompanying the isomerization and reversion result in transmembrane H+ movement. Bacteriorhodopsin as a model for mechanisms by which proton pumping is coupled to exergonic electron transfer reactions in the ETC.
Bacteriorhodopsin is a light-driven proton pump. This 248-residue integral membrane protein from Halobacterium salinarum (Halobacterium halobium) provides an excellent model (mainly in the sense of being the best understood model and relatively simple in its mechanism) for how cells use available energy to actively transport protons (H+) across a membrane, thereby converting the original energy source into the form of a transmembrane proton gradient. Although the energy to drive the proton pumping by the membrane protein complexes of the electron transport chain in eukaryotic mitochondria is provided by redox reactions, not light, similar principles appear to apply to the proton translocation nechanism.
Structures. PDB entries for bacteriorhodopsin:
Bacteriorhodopsin.
Molecule of the Month (March 2002).
1fbb -
Crystal structure of native conformation of bacteriorhodopsin,
determined by electron crystallography to 3.2 Å resolution
1c8r -
Bacteriorhodopsin D96N BR state at 2.0 Å resolution
1c3w -
Bacteriorhodopsin/lipid compex at 1.55 Å resolution,
determined from crystals grown in cubic lipid phase
1dze -
Structure of the M intermediate of bacteriorhodopsin trappped at 100 K (2.5 Å resolution)
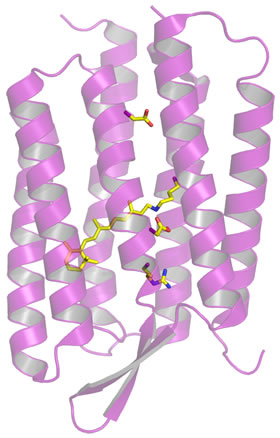
The structure of bacteriorhodopsin consists for the most part of seven transmembrane helices (figure at right). This seven-helix tertiary structure is a common protein fold for integral membrane proteins. Bacteriorhodopsin in fact serves as a paradigm for the family of integral membrane proteins that includes G-protein-coupled receptors (GPCRs; examples of GPCRs are rhodopsin and adrenergic receptors). There is a polar channel through the center of the structure that also contains a retinal prosthetic group. The retinal is covalently attached to Lys216, via a Schiff base linkage.
The figure below represents the light-driven trans to cis isomerization,
and its spontaneous reversion.
When the retinal prosthetic group, in its ground-state
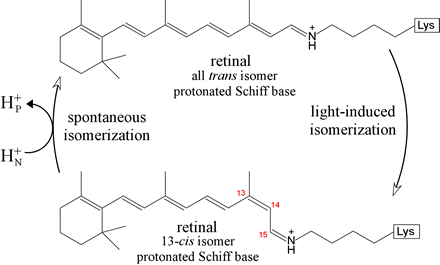
Note the highly conjugated system of double bonds of the retinal Schiff base forms. The energy levels of electronic states become more closely spaced in such systems, hence bacteriorhodopsin's retinal absorbs light energy of a longer wavelength. The isomerization of retinal changes the location of the Schiff base group, and its changed environment alters its pKa. This allows the protonated form to transfer its proton across the membrane. A combination of spectroscopic and structural approaches supports a mechanism for the light-driven isomerization consisting of a series of states that account for this proton pumping.
Related topics pages: