BIOCHEMISTRY TOPICS
Protein structure: The α helix
The regular, ideal alpha helix conformation and its characteristics. Helical wheels and amphipathic α helices. Heptad repeats and coiled-coils. Other helical conformations. Fibrous proteins.
Secondary structures: the α helix and β strand conformations
Polypeptide chains can adopt regularly repeating main chain conformations. Such conformations are generally the most stable (energetically favorable). One of these conformations, proposed first by Pauling and in fact observed prominently in protein tertiary structure, is the α helix (alpha helix, model at right).
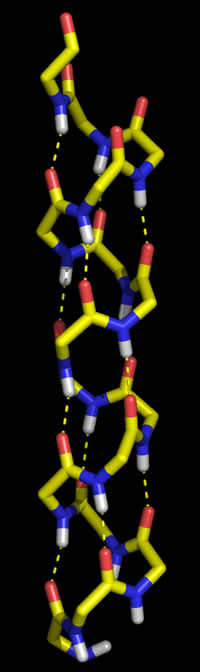
Right: Molecular graphics image of 16 residues of poly Gly in the α helix conformation. In the stick representation, atoms are at ends and vertices, and yellow indicates carbon, red oxygen, blue nitrogen, and white hydrogen (Only main chain amide hydrogen atoms are shown). Hydrogen bonds are indicated by the dashed yellow lines, and form between the acyl oxygen of residue i (acceptor) and the amide group of residue i + 4 (donor).
The other regular conformation is the β strand conformation, Together with turns and loops, the α helix and β strand could be likened to "elements" that are put together to form higher-order "compounds" of of protein structure.
Characteristics of the α helix
The ideal α helix has the following features:
- Right-handed
- 3.6 residues per turn
- Pitch = 5.4 Å
- H-bond distances (amide N to acyl O) ~ 2.8 Å
- In proteins, an average length is ~ 12 residues (~ 18 Å)
"Helical wheel" diagram and amphipathic helices
An isolated α helix is more the exception rather than the rule - in other words, α helices are usually found in association with other structural "elements". For example, two α helices may associate due to favorable contacts between side chains. To help us better understand this and other structural associations involving α helices, we make use of helical wheel diagrams.
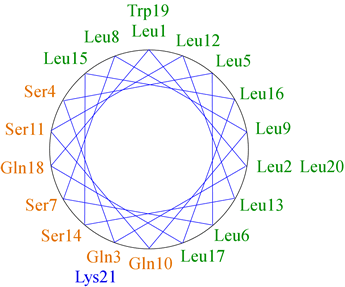
The figure at left shows the positions of the side chains along a regular α-helix. For a right-handed helix with 3.6 residues per turn, rotation is clockwise as the polypeptide chain is followed N to C, and the 5th residue ends up in a position 40° clockwise relative to residue 1. Note that over a number of turns of the helix (21 residues or nearly six turns of the helix), a pattern of distribution of the side chains emerges in this example. Nonpolar side chains group on one side of the helix, whereas polar and charged side chains are grouped to the other side. This gives the helix an amphipathic character, having obvious implications for the orientation of such a helix in the context of tertiary structure. The nonpolar face of the helix can interact favorably with (and form part of) the nonpolar core of a globular protein, while the hydrophilic face will orient toward the surface.
As the example of an amphipathic regular α helix above suggests, the interaction of an α helix with other elements of protein structure occurs mainly through its side chain contacts. A specific example of this is the coiled coil motif, which occurs in many structural proteins such as keratin and an important class of transcription factors known as bZIP transcription factors. If an α helix is slightly distorted so that it has 3.5 residues per turn, then every seventh side chain will emanate from the same angular position on the helix surface (translated, of course, parallel to the helix axis, by 10 Å or so). This is known as a heptad repeat, and the energy required to distort the helix from the ideal geometry is made up for by favorable interactions between side chains in two or more such helices.
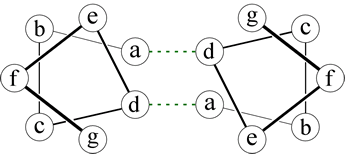 Right: A helical wheel diagram for two heptad repeats that form a coiled-coil. The dashed lines indicate packing interactions involving the side chains at the a and d positions.
Right: A helical wheel diagram for two heptad repeats that form a coiled-coil. The dashed lines indicate packing interactions involving the side chains at the a and d positions.
A dimeric interaction (between two helices) is mediated by nonpolar side chains in two positions of the respective heptads, as shown in the figure. The heptad positions are labeled a - g, by convention, and it is the residues at positions a and d that form favorable contacts. The distortion of the two helices results in the formation of a superhelix - that is, the two helices wind around each other - hence the name "coiled-coil" for this type of motif.
Related topics pages: |