BIOCHEMISTRY TOPICS
Regulation of glycolysis
Overview. Regulation of phosphofructokinase (PFK) activity: Allosteric effects of ATP and AMP.
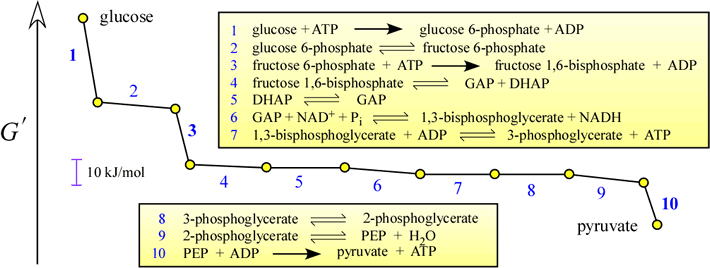
Regulation of glycolysis occurs at three points of the pathway. These correspond to the steps with the largest negative free energy changes (i.e. most exergonic - negative ΔG). The magnitude of the ΔG for these steps makes them essentially irreversible. The most important point of control is at the reaction catalyzed by phosphofructokinase (PFK, Reaction 3, EC 2.7.1.1]. Other control points are the hexokinase (Reaction 1) and pyruvate kinase (Reaction 10) reactions.
Regulation of PFK
The reaction catalyzed by PFK is the committed step of glycolysis. The committed step of the pathway is defined as the first highly exergonic step that is unique to that pathway. PFK would seem to be a logical choice for regulation, and indeed PFK displays allosteric regulation. ATP is an inhibitor (as well as a substrate!) of PFK. In many eukaryotic PFK orthologs, a "side" metabolite of glycolysis, fructose 2,6-bisphosphate, activates the enzyme. Furthermore, fructose 2,6-bisphosphate relieves the inhibitory effect of ATP (see Ref.2, Figs.16.16 and 16.18, p.453, 455).
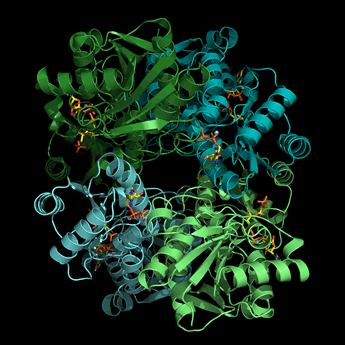
The structure of PFK from E. coli is formed as a homotetramer (each monomer is a different color in the ribbon diagram of PFK at left, shown in a view similar to that of Fig.16.15 in Ref.2) with distinct catalytic and allosteric sites, both located near subunit interfaces. Homoterameric PFK enzymes from a variety of organisms are inhibited by ATP and citrate and activated by AMP. A high energy charge would likely slow glycolysis by inhibiting PFK, while a low energy charge should favor increased glycolytic flux by activating PFK.
Left: Image of the structure of PFK from E. coli (generated from pdb id 1pfk). Each monomer of this tetrameric enzyme, represented in ribbon form, is shown in a different color. The bound products (ADP + fructose 1,6-bisphosphate) and effectors (ADP) are shown in stick form.
The allosteric behavior of PFK shows up first of all in its positive cooperativity in rate with respect to its substrate fructose 6-phosphate (F6P). The graphs of PFK velocity vs. F6P concentration show sigmoidal kinetics. ATP is of course also a substrate, but the data suggest that ATP must be a heterotropic inhibitor as well.
Although PFK homologs from a variety of organisms and tissues show a corresponding variety in regulatory properties, they all seem to share the inhibition by high levels of ATP. Interestingly, AMP is quite effective at reversing inhibition of PFK by ATP. One-tenth the concentration of AMP relative to ATP increases the rate of PFK by five-fold under conditions with [F6P] = 0.5 mM. The concentration of ATP in cells is buffered by the actions of creatine kinase and adenylate kinase, and its levels are thought to vary between rest and vigorous activity by no more than 10% (in muscle tissue, for example). However, AMP levels, while much lower, vary much more dramatically, and therefore may serve as a more effective regulatory signal than ADP.
While AMP may be a pretty good in general at countering ATP inhibition of PFKs, another allosteric regulator of PFK from animals, fructose 2,6-bisphosphate, is an even more potent activator. PFK from mammalian liver is allosterically activated by fructose 2,6-bisphosphate, as shown by a graph comparing the initial rate of PFK versus substrate concentration with and without fructose 2,6-bisphosphate (e.g. Ref.2, Fig.16.18, p.455). The velocity vs substrate concentration curve changes from sigmoidal to hyperbolic in form, as if fructose 2,6-bisphosphate binding were coupled to the complete transition of the enzyme to an R state. Fructose 2,6-bisphosphate also relieves the inhibitory effect of ATP.
Fructose 2,6-bisphosphate controls the activity of PFK, but what produces and controls levels of fructose 2,6-bisphosphate? A kinase, "PFK2", phosphorylates the 2-OH of fructose 6-phosphate, yielding fructose 2,6-bisphosphate. PFK2 activity is stimulated by fructose 6-phosphate - a "feed-forward" control feature. The activity of PFK2 turns out to reside within the same polypeptide as the phosphatase activity ("FBPase2") that hydrolyzes the 2-phosphate of fructose 2,6-bisphosphate. In mammalian liver, the activity of this bifunctional protein is switched from kinase to phosphatase activity due to phosphorylation of it by protein kinase A (PKA) at a single serine residue in the N-terminal regulatory domain. The protein kinase activity is stimulated by low blood glucose due to an increase in intracellular levels of cyclic AMP (cAMP). This control mechanism operates in hepatocytes (liver cells) to slow down glycolysis when blood sugar levels are low.
Regulation of fructose 2,6-bisphosphate levels by PKA
Fructose 2,6-bisphosphate has a activating effect on the activity of PFK, but what produces and controls levels fructose 2,6-bisphosphate itself? The levels of fructose 2,6-bisphophate levels are directly controlled by the activity of a kinase, "PFK2", that phosphorylates the 2-hydroxyl group of fructose 6-phosphate, as well as the opposing activity of a phosphatase ("FBPase2"), that promotes the hydrolysis of the 2-phosphoryl group. Remarkably, the activity of PFK2 turns out to reside within the same polypeptide as the FBPase2 phosphatase activity. The activity of this bifunctional protein (PFK2/FBPase2) is switched between kinase and phosphatase activity according to phosphorylation of a single serine residue in an N-terminal regulatory domain. The phosphorylation of this serine is the result of the action of protein kinase A (PKA). In the liver, when glucose is abundant, PKA is elevated. Whether the bifunctional protein is phosphorylated or not is regulated in large measure by PKA and protein phosphatase activity (see Ref.2, Fig.16.30, p.467). Recall that PKA, activated by cyclic AMP (cAMP) (Ref.2, Ch.10, pp.287-288), is part of a signal transduction pathway (see Ref.2, Ch.14, pp.383b-386) initiated by the binding of an extracellular hormonal signal to a G protein-coupled receptor (GPCR), leading to activation of a membrane-associated adenylate cyclase.
When glucose is abundant, glycolysis tends to be more active. If levels of fructose 6-phosphate increase, phosphoprotein phosphatase activity is stimulated (this feedforward stimulation is indicated in Ref.2, Fig.16.30 by the dashed-line curved arrow). The protein phosphatase may also be stimulated in cells responsive to insulin signaling. The increased protein phosphatase activity favors PFK2 activity, increasing levels of fructose 2,6-bisphosphate, stimulating PFK (PFK1) activity.
When glucose is scarce, PKA will be activated in cells responsive to glucagon, which favors FBPase2 activity, lowering levels of fructose 2,6-bisphosphate. The loss of the PFK activation by the latter slows down glycolysis. In liver, the effect of glucagon is also to stimulate glycogen breakdown, thus making the glucose stored therein available for maintenance of blood-glucose homeostasis.
Regulation of other glycolytic enzymes
The other points at which the flux through the glycolytic pathway can be controlled include the activities of hexokinase and pyruvate kinase. Hexokinase is subject to product inhibition by glucose 6-phosphate. When PFK is less active, the rise in relative concentration of fructose 6-phosphate is soon reflected in a rise in glucose 6-phosphate levels. This also slows the rate of catalysis by hexokinase. In the liver, this mode of regulation can be bypassed as glucose 6-phosphate levels rise by the enzyme glucokinase. Glucokinase is not inhibited by G6P, but its KM for glucose is significantly higher.
Regulation of pyruvate kinase occurs via allosteric effects, and through different isozymic forms that differ in their capacity for regulation by covalent modification (again, phosphorylation). In general, fructose 1,6-bisphosphate, the product of the PFK reaction, is an allosteric activator of pyruvate kinase, while ATP and alanine (the latter signifies the abundance of pyruvate) are allosteric inhibitors.
The L isozyme of pyruvate kinase is directly regulated by phosphorylation. The L form is expressed in the liver, and it is a substrate of PKA When blood glucose is low, glucagon stimulates a membrane associated adenylate cyclase, activating PKA, as explained above. This leads to the phosphorylation of the L form of pyruvate kinase, which inhibits its activity. This makes metabolic sense, since when blood glucose is low, further consumption of glucose by glycolysis in the liver ought to be slowed down.
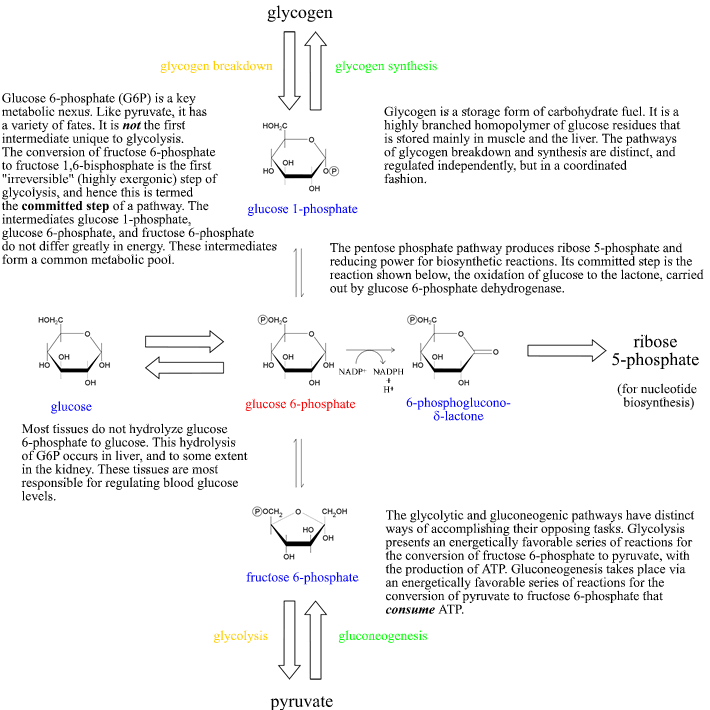
Glycolysis in context: Glucose 6-phosphate at the crossroads
The fate of glucose 6-phosphate (G6P) is not determined solely by the rate of glycolysis. It is also utilized in the pentose phosphate pathway, or it can be directed toward "short-term storage" in the form of glycogen. In considering the logic of metabolism in complex organisms, the specialized roles of organs must be included. The liver, in its role as a regulator of blood glucose levels, carries out the hydrolysis of G6P to glucose for release into the bloodstream. Below is a summary of the fates of G6P in a broader overview of its relation to major metabolic pathways. "Irreversible" parts of pathways are represented by the large arrows. The oxidation of G6P to 6-phosphoglucono-δ-lactone (the first step of the oxidative branch of the pentose phosphate pathway) is also an irreversible step, as indicated by the single arrow pointing to the right. Note that glucose 1-phosphate, G6P, and fructose 6-phosphate are interconvertible in reactions that are not highly exergonic (or endergonic), and thus in a sense constitute a common metabolic pool. We can thus think of these as a common pool of hexose monophosphates, and the direction of "flow" among them determined by the particular metabolic or physiological situation.