BIOCHEMISTRY TOPICS
Glycolysis: Reactions and enzymes
The glycolytic pathway, step-by-step.
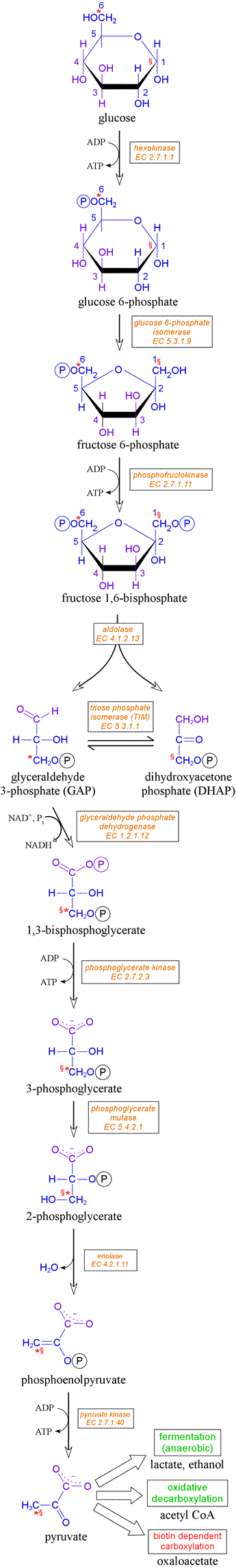
Glycolysis is an energy-producing, catabolic pathway that can be considered as the breakdown of the six-carbon sugar glucose to two 3-carbon end-products, with a net yield of two high-energy phosphoanhydride bonds of ATP and two reducing equivalents in the form of NADH per glucose molecule consumed. The exact end-products of glycolysis depends on whether it occurs under aerobic or anaerobic conditions. In the former case, the end product is pyruvate, which is subsequently oxidatively decarboxylated by the multienzyme pyruvate dehydrogenase (PDH) complex. In the anaerobic case, the requirement for the recycling of NADH to NAD+ must be met by the reduction of pyruvate - for example to lactate - which constitutes the process of fermentation.
The names of the enzymes and the names and structures of intermediates in the glycolytic pathway, beginning from glucose and ending with pyruvate, are shown at right. The phosphorylation of glucose to form glucose 6-phosphate is carried out by the enzyme hexokinase [EC 2.7.1.1]. Glucose 6-phosphate is a key metabolic branch point, meaning it has a number of possible fates. However, its transformation to fructose 6-phosphate is the next step of glycolysis. The reaction is catalyzed by the enzyme glucose 6-phosphate isomerase [EC 5.3.1.9; also called phosphoglucose isomerase]. A key step in the control of glycolysis follows, a second phosphorylation of fructose 6-phosphate produceing fructose 1,6-bisphosphate(FBP). The activity of the enzyme for this step, phosphofructokinase (PFK) [EC 2.7.1.11], is highly regulated and exerts the most influence on the overall rate of glycolysis.
The next stage of glycolysis commences with the enzyme aldolase catalyzing the cleavage of the six-carbon FBP into two three-carbon phosphosugars, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP). From this point onward, all the intermediates of glycolysis are three-carbon compounds. The enzyme triose phosphate isomerase (TIM) [EC 5.3.1.1] ensures rapid equilibration between the ketose DHAP and the aldose GAP. Only the latter progesses further along the glycolytic pathway, so that the combined action of aldolase and TIM can be considered to be the conversion of FBP into two molecules of GAP.
GAP is then oxidized and phosphorylated in a remarkable reaction catalyzed by the enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The substrates are GAP, NAD+, and inorganic phosphate (Pi). The products are NADH and 1,3-bisphosphoglycerate. The latter product of the GAPDH reaction is an acyl phosphate compound, which donates its phosphate group in the next reaction to ADP, forming ATP and 3-phosphoglycerate. This reaction, catalyzed by phosphoglycerate kinase [EC 2.7.2.3], is the first of two substrate-level phosphorylations by which biochemical energy in the form of ATP is harvested in this energy-yielding phase of the glycolytic pathway.
Under anaerobic conditions, and inasamuch as the pool of nicotinamide cofactor is limited, the NAD+ consumed in the GAPDH reaction must be regenerated for glycolysis to continue. This is the purpose of fermentation.
In order to set the stage for the next ATP-yielding step and the conclusion of the glycolytic pathway, 3-phosphoglycerate must first be isomerized to 2-phosphoglycerate by the activity of phosphoglycerate mutase [EC 5.4.2.1]. Next, phosphoenolpyruvate (PEP) is formed from 2-phosphoglycerate in a reaction catalyzed by enolase. Finally, the second substrate-level phosphorylation takes place via the pyruvate kinase [EC 2.7.1.40] reaction, in which the substrates ADP and PEP yield the products ATP and pyruvate.
The figure showing the pathway, from glucose to pyruvate, also indicates the fate of individual atoms. For instance, carbons 1 and 6 of glucose (marked as red § and * characters, respectively) both appear as carbon 3 of pyruvate. Radiolabeling experiments have been a primary method in elucidating and confirming biochemical pathways and the enzymatic mechanisms of individual steps.
The product of the common glycolytic pathway is pyruvate. There are a number of alternate fates for pyruvate, and some major fates are indicated at right. Under anaerobic conditions, fermentation occurs in order to regenerate NAD+ for continued glycolysis. In human muscle exercising vigorously enough, the rate of oxygen consumption cannot keep up, and the muscle operates anaerobically. In this case pyruvate is reduced to lactate by lactate dehydrogenase [EC 1.1.1.27]. The lactate produced by muscle is exported to the liver via the bloodstream, and in the liver lactate is re-oxidized to pyruvate. This is half of what is known as the Cori cycle. In microbiology, the biochemistry of fermentation is diverse, but includes lactate production, as well as a non-oxidative decarboxylation of pyruvate, followed by reduction of acetaldehyde to ethanol, the latter catalyzed by alcohol dehydrogenase [EC 1.1.1.1].
In aerobic metabolism, the principal fate of pyruvate is its oxidative decarboxylation, coupled to the formation of acetyl CoA, carried out by the pyruvate dehydrogenase (PDH) complex.
A third major fate for pyruvate is carboxylation to form oxaloacetate. The biotin-dependent carboxylase that catalyzes this reaction is pyruvate carboxylase [EC 6.4.1.1]. In aerobic metabolism, some oxaloacetate production is required to keep it balanced with acetyl CoA for the continued metabolic flux through the citric acid cycle. In other circumstances, pyruvate carboxylation serves as the first step of regeneration of glucose by gluconeogenesis. This would not be the case for cells or tissues actively carrying out glycolysis, but one case would be that of the liver of a person who has exercised vigorously enough to incur some oxygen debt. Having accepted muscle-generated lactate, the liver reoxidizes it to pyruvate, from which it regenerates glucose by gluconeogenesis. The liver exports this glucose to the bloodstream for use by muscles for continued work. This is the other half of the Cori cycle.
Mechanisms of glycolytic enzymes
aldolase - protonated Schiff base intermediate
The mechanism of a Class I aldolase begins with formation of a Schiff base linkage between enzyme (amine group from a lysine side chain) and substrate (keto group from open chain form of fructose 1,6-bisphosphate (FBP). The protonated Schiff base acts as an electron sink to stabilize the negative charge that develops with carbon-carbon bond cleavage. Comparing the mechanism of aldolase to that of a base-catalyzed aldol cleavage reaction can help us understand its fundamental nature.
TIM - triose phosphate isomerase - enzymatic catalysis: "not different, just better"
The isomerization reaction catalyzed by this enzyme interconverts the ketose dihydroxyacetone phosphate (DHAP) and the aldose glyceraldehyde 3-phosphate (GAP) via a enediol intermediate. We easily envision how such a reaction can be catalyzed by general-acid and general-base catalysis. This is in large measure exactly how TIM works, but the actual mechanism incorporates a surprising twist on this simple scheme.
GAPDH - glyceraldehyde 3-phosphate dehydrogenase - a thioester intermediate
In this mechanism, the energy releasing process of oxidation of the aldehyde group of glyceraldehyde 3-phosphate to a carboxylate group is coupled to the energy-requiring step of forming an acyl phosphate from a carboxylate group. The coupling is achieved via the device of a thioester formed by nucleophilic attack by a specific cysteine residue in GAPDH on the carbonyl carbon of glyceraldehyde 3-phosphate.
The regulation of the glycolytic pathway also has a logic, one that is context-dependent. We finish our study of glycolysis with a look at some control issues. In order to fully understand the regulation of glycolysis, we must know a little bit about its alter-ego pathway, gluconeogenesis.