GENERAL CHEMISTRY TOPICS
Atoms: An introduction
Models for the atom. Subatomic particles. The electron shell model. The shell model and chemical periodicity.
Atomic theory: The beginnings
One of the major themes of general chemistry is the correlation of observable properties of substances that we observe on a macroscopic scale with the nature the constituents of matter on the on the ultra-microscopic scale. In fact, the structures and behavior of these constituents - atoms and molecules - provide a model upon which explanations and predictions for our observations of the chemical and physical properties of substances in the laboratory are based. This is what a good scientific theory does of course: it serves as a powerful explanatory principle. We need to start our study of chemistry with a basic understanding of the major features of atomic theory, including some of the most significant developments of 20th century science.
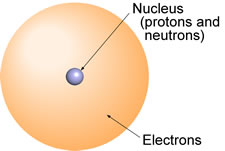
Most of us have been exposed to enough science to know that atoms - as shown in the illustration - are composed of a nucleus, which contains protons and neutrons, while the bulk of the actual "volume" of an atom is somehow occupied by a "cloud" of electrons surrounding the nucleus. The simplest atom is the hydrogen atom, which consists of a single proton as its nucleus, and a single electron.
We likely have a sense that there are many different types of atoms, and that these typically combine in various ways to form stable arrangements of atoms called molecules. Indeed this is - as suggested above - what chemistry is fundamentally all about. We want to learn the answers to questions such as: Why do atoms combine to form molecules, how are the atoms in a molecule arranged in space, and how do these arrangements determine the properties of substances that we observe in the macroscopic realm? In other words, we want to know the how and why of chemical bonds, what the molecular structures (or strictly speaking, models of molecular structure) look like, and how molecular structure influences the bulk properties of matter. As a preview of what form the answers we seek will take in terms of atomic theory, it can be said that it is the electrons of atoms that play the predominant role in chemical bonding and molecular structure, with the properties of nuclei influencing the numbers of electrons atoms have, as well as the patterns of their distribution about the nucleus. We will learn much more about atomic theory and electron configurations later in the course. We will also investigate the nature of chemical bonds and examine molecular structure. Subsequently, we will relate our understanding of molecular structure to the macroscopic properties of substances.
Models for the atom
Before any experimental evidence, the existence of atoms was posited by ancient philosophers as a consequence of the idea that there must be some limit to the divisibility of matter into smaller and smaller portions. Democritus (466-370 BCE) is usually credited with developing the concept during a period in which classical Greek civilization gave rise to an interest in natural philosophy. Democritus' atoms (the term was derived from the Greek atomos, meaning "uncuttable") were solid, indivisible, and indestructible. Furthermore, the atoms could stick together upon collision, and such agglomeration of atoms gave rise to the variety of substances observed in the world3, 4. Remarkably, these speculative propositions bore a striking resemblance to Dalton's atomic theory, put forward 1200 years later. As chemistry developed in the 18th century, indirect evidence began to accumulate for the existence of atoms and for the proposition that the variety of pure substances is a result of the tendency of atoms to form different stable combinations in definite proportions. In other words, atoms combine to form molecules, which are thus the smallest unit of the pure substances known as compounds.
By the first decade of the 19th century, the English scientist John Dalton had proposed the first recognizably modern version of atomic theory. Let's outline the development of atomic theory, and in so doing we will arrive at a model for the atom that will prove to be of great utility in understanding much of chemistry.
Dalton's atomic theory of matter. Dalton's postulates4 were as follows:
- All matter consists of solid and indivisible atoms
- All atoms of a given element are identical
- Different elements have different kinds of atoms, differing in mass
- Atoms are indestructible
- Compounds are formed by combination of different atoms in small proportions
As we'll see, postulates 1, 2, and 4 would need to be revised in the light of later discoveries, but Dalton's theory was a major advance.
The Rutherford model. The indivisibility of atoms was the first of Dalton's postulates to require revision. Observations and experiments throughout the 19th century suggested atoms were made up of smaller particles. The electron, a negatively charged particle very much smaller than the atom, was the first to be described and measured. J. J. Thomson (1856-1940), through his investigation of cathode rays, discovered that these "rays" carried electric charge and proposed that they were made up of tiny "corpuscles", for which Thomson was able to measure a charge-to-mass ratio. Thomson's negatively charged corpuscles, later named electrons, were found to be constituents of all atoms. Since atoms themselves are electrically neutral, there must also be some positively-charged component to balance the charge within the atom. This led Thomson to propose the whimsically named "plum pudding" model for the atom. In this model, the atom was a sphere of positively-charged material within which the requisite number of much smaller electrons were embedded, like raisins in plum pudding. The demise of the plum pudding model was due to the handiwork of Thomson's student Ernest Rutherford (1871-1937). The results of experiments in Rutherford's lab investigating the deflection of alpha particles directed at a thin piece of gold foil were wildly inconsistent with the plum pudding model. Specifically, the trajectory of positively-charged alpha particles (which are helium nuclei resulting from radioactive decay) would be expected to be relatively unaffected by an array of plum-pudding gold atoms. However, a small proportion of the alpha particles ricocheted as if they had encountered a massive subatomic object. Rutherford described his astonishment in vivid terms, stating that it "was almost as incredible as if you fired as 15-inch shell at a piece of tissue paper and it cam back and hit you." 6 This led him to propose the first nuclear model for the atom. In Rutherford's model, most of the mass and positive charge of an atom is centrally concentrated in a nucleus. In contrast, electrons were somehow diffusely distributed around the nucleus to define the size of the atom, but overall, most of the atomic volume was simply empty space. The nucleus is on the order of 10−15 m and an atom is on the order of 10−10 m in size. In visualizing the relative scales of nuclear and atomic sizes, images such as the "fly in the cathedral" or the "pea in the stadium" are commonly invoked. Subsequently, Rutherford showed that the nucleus of the hydrogen atom (the smallest and simplest nucleus) consisted of a particle with a unit of positive charge (exactly equal but opposite to the electron), and which makes up the positive charges of all other nuclei. Rutherford named this particle the proton. Later, the neutron was discovered as a second nuclear component, with a mass approximately equal to the proton (electrons are more than 1800 times lighter than protons and neutrons). All nuclei were determined to be made up of protons and neutrons.
At this point, let's summarize the properties of the subatomic particles that make up the atom in the following table.
Table 1: Subatomic particles |
||||||
| Particle | Symbol | Mass (g) | Mass (amu)* | Charge† | ||
| Proton | p+ | 1.672623 × 10−24 . | 1.00728 | 1 + | ||
| Neutron | n | 1.674929 × 10−24 . | 1.00866 | 0 | ||
| Electron | e− | 9.10939 × 10−28 . | 5.48680 × 10−4 | 1 − | ||
| * Atomic mass unit, amu is defined as exactly 1/12 the mass of a carbon-12 atom; 1 amu = 1.660539 × 10−24 g. † Charges are given in elementary charge units, e = 1.602176 × 10−19 C (coulomb, C), which is the charge of an electron. |
||||||
The electron shell model
This model can be thought of as building on Rutherford's by describing the distribution of electrons about the nucleus. Due to electrostatic potential energy, the electrons in an atom all experience an attractive force due to the positive charge of the nucleus, and simultaneously there is a repulsive force between electrons. In the electron shell model (or as we'll on occasion refer to more simply as the shell model), the electrons are organized into shells - analogous to the layers of an onion - with the electrons in shells close to the nucleus referred to as core electrons, and those in the outermost shell are called valence electrons. The electron shell model is a chemical model for the atom inasmuch as it is the valence electrons of various elements that greatly influences their reactivity.
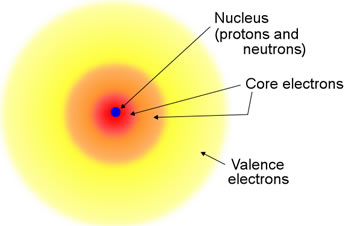
Left: Diagram of the shell model, showing the coarse internal structure of the atom. At the center is the dense nucleus, containing the protons and neutrons, and accounting for nearly all of the mass of an atom. Surrounding the nucleus are the diffusely distributed electrons, which are pictured as organized into shells. The core electrons are those in the innermost shells and are found for the most part close to the nucleus. The electrons in the outermost shell are the valence electrons. Note the size of the nucleus relative to the atom is vastly exaggerated in this figure.
The valence electrons are located much further on average from the nucleus, so they would be less strongly attracted to it. Valence electrons experience less of an attractive electrostatic force than core electrons. This is usually explained by means of a sheilding effect by core electrons occupying the inner shells, so that the effective nuclear charge for a valence electron is less than the full nuclear charge. As a further consequence, valence electrons are available to participate as electron donors, sharing in chemical bonding between atoms in molecules and molecular ions. The electron shell model is quite successful at explaining the chemical bonding behavior of different atoms by focusing attention on the valence electrons.
The shell model and chemical periodicity. A shell model for how the electrons are organized within atoms would seem to suggest several features.
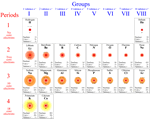
First, the electrons within any given shell would all be located at about the same distance from nucleus - at least on average. Second, this average distance is significantly different for each shell, increasing as the number of shells increases. This follows from what we already noted for valence electrons, the outermost shell being furthest from the nucleus. Third, as shells are added in order to accommodate the electrons in atoms of larger atomic number, the number of electrons that can fit in a shell should increase.
These features can account in general for the structure of the periodic table and the periodic variation in atomic sizes (see chart showing elements 1-20). The structure of the periodic table is consistent with a first, innermost shell that can hold only two electrons, a second and third shell that hold eight electrons each, a fourth and fifth that hold 18 electrons each, and so on. If we look at the sizes of atoms (these can be experimentally determined by a technique called X-ray diffraction) we see that the atoms of elements in period 2 are much larger than those in period 1, and significantly smaller than those of period 3. Interestingly, atomic size actually decreases within a period, from left to right. Does the shell model offer a possible explanation? (Hint: Consider the effects of electrostatic potential energy.)
Another important feature of the electron shell model that is not immediately apparent is that filled shells are a stabilizing factor for atoms and ions. This is a necessary postulate to address the remarkable lack of chemical reactivity observed for the Group VIII elements, the noble gases. While this is a rather ad hoc postulate, it is a useful one for rationalizing chemical properties, even of elements other than noble gases. In this view, elements exhibit a strong tendency to combine chemically in ways such that each atom within the molecules or ions formed attains a filled-shell electron configuration, like that found in isolated atoms of a noble gas. Atoms achieve this by gaining, losing, or sharing valence electrons. For instance, the chemistry of Group I and VII elements is largely accounted for by the loss and gain, respectively, of one electron to become ions. In either case, the ion formed has filled shell electron configuration.
The quantum mechanical atom. The most modern model, the quantum mechanical view of the atom accounts for the wave-like properties of subatomic particles. In particular, this model explains why the electrons in atoms behave as if they were organized into shells.