GENERAL CHEMISTRY TOPICS
Ionic and net ionic equations
Symbolic representations of ionic species as chemical formulas, and reactions involving ionic species in chemical equations, with particular focus on the formation and reactions of ions in aqueous solutions. Dissolution equation for a water-soluble ionic compound.
Once we begin to consider aqueous solutions and encounter the phenomenon of electrolytes, our symbolic representation of solute species and the reactions involving them must necessarily incorporate solvated ionic species.
Let's begin with the dissolution of a water soluble ionic compound. In this case, the solid ionic compound dissolves and completely dissociates into its component ionic species, which are homogeneously dispersed throughout the bulk aqueous solvent. So how should a chemical equation be written to represent this process?
Using the familiar compound sodium chloride as an illustrative example, we can write the formula NaCl along with the label ("s") to specifically represent the solid form of the compound. If we then take a small sample of the salt and combine it with a larger amount of pure water, the salt (which we denote as the solute) dissolves in the water (denoted the solvent) to form a homogeneous mixture, indistinguishable in appearance from the initial pure water, that we call the solution. Let's discuss how the dissolution process is represented as a chemical equation, a dissolution equation for a water soluble ionic compound.
Since the solid sodium chloride has undergone a change in appearance and form, we could simply represent this symbolically by replacing the appended "s" label with "aq". The latter denotes a species in aqueous solution, and the first equation written below can be read as "solid sodium chloride combined with a superstoichiometric amount of water (solvent) becomes an aqueous solution of sodium chloride."
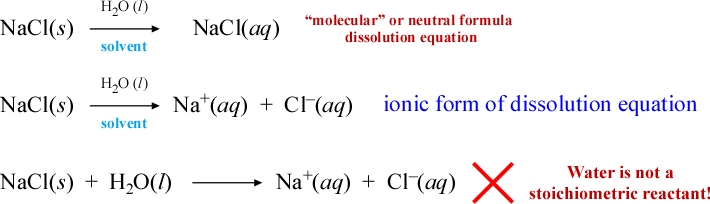
The term we'll use for this form of the equation representing this process is the neutral formula (or "molecular") dissolution equation. Why is water not written as a reactant? Do we really know the true form of "NaCl(aq)"? Has a chemical reaction occurred or is dissolution of salt a merely physical process?
First of all, the key observation is that pure water is a nonelectrolyte, while the conductivity of the sodium chloride solution shows that the solute is a strong electrolyte. This is strong evidence for the formation of separated, mobile charged species in solution. This is represented by the second equation showing the explicit formation of aqueous forms of sodium cation and chloride anion. Water is not written as a reactant because we are viewing the solvent as providing only the bulk environment for solution formation. It is true that at the molecular level there are significant ion-dipole interactions between the ions and nearby water molecules, and a variety of solvated species that can be described as an ion surrounded by a stoichiometric number of water molecules in a "solvation shell" have been revealed experimentally. However we'll let symbols such as "Na+(aq)" represent collectively all solvated ionic species in aqueous solution. Thus inclusion of water as a reactant is normally unwarranted, although as an unbalanced "skeletal" chemical equation it is not wildly out of place.
Is the dissolution of a water-soluble ionic compound a chemical reaction? The fact that the ionic bonds in the solid state are broken suggests that it is, and highlights the favorable effect of solvation and dispersal of ions in solution. On the other hand, the dissolution process can be reversed by simply allowing the solvent water to evaporate.
The advantage of the second equation above over the first is that it is a better representation of the existence of separated charged species, that the solute is an electrolyte. A neutral formula unit for the dissolved species obscures this fact, and for water-soluble ionic compounds at an atomic scale, "molecules" such as NaCl are not present to any significant extent. The ionic form of the dissolution equation is our first example of an ionic equation.
Other examples of dissolution equations for water-soluble ionic compounds, such as the one for lead(II) nitrate shown at left highlight the accompanying stoichiometric relationships. The equation can be read as one neutral formula unit of lead(II) nitrate combined with a superstoichiometric amount of water (solvent) yields one lead(II) cation and two nitrate anions, both ions in aqueous phase. In case of hydrates, we could show the waters of hydration as product species. This would be correct stoichiometrically, but such product water molecules can be dropped from the dissolution equation if they are considered indistinguishable from bulk solvent molecules once released from the solid phase structure.
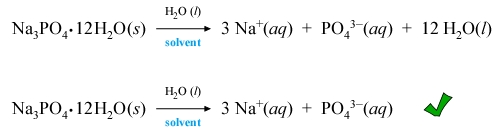
Strictly speaking, this equation would be considered unbalanced. In writing it as shown we are treating waters of hydration as part of bulk solvent on the product side.
Equations for ionic reactions
Let's now consider a number of examples of chemical reactions involving ions. In the context of the examples presented, some guidelines for writing such equations emerge.
In writing the dissolution equation, it is assumed that the compound undergoing dissolution is indeed soluble in water and that the product solution is not saturated. When saturation is reached, every further amount of solute added to the system results in the appearance and accumulation of undissolved solid. This creates the potential for the reverse of dissolution, formally a precipitation reaction, and sets up a dynamic equilibrium between the two opposing processes. Symbolically, the condition or potential for dynamic equilibrium is represented by replacement of the forward arrow with the double single-barbed arrow symbol (as shown in figure).
The equation representing the solubility equilibrium for silver(I) sulfate.
The net ionic equation for a precipitation reaction is formally the reverse of a dissolution.
The formation of stable molecular species such as water, carbon dioxide, and ammonia.
See also the discussion and the examples provided in the following pages: precipitation and acid-base reactions, introduction to chemical equations.