Lecture 31. Citric acid cycle
Monday 28 November 2016
The reactions and enzymes of the citric acid cycle. Citrate synthase - structure, function, and mechanism. Aconitase. Isocitrate dehydrogenase (IDH): Oxidative decarboxylation of a β-keto acid. The α-ketoglutarate dehydrogenase complex. The stereochemistry of the citric acid cycle and hydride transfer to NAD+. Succinyl CoA synthetase: Mechanism and structure. Regeneration of oxaloacetate. Stoichiometry of the citric acid cycle. Regulation of the citric acid cycle.
Reading: VVP4e - Ch.17, pp.561-572.
Summary
Reactions of the citric acid cycle
Four of the six total CO2 produced per glucose arise from two turns of the cycle.
Although it yields very little energy in the form of high-energy phosphates, the citric acid cycle is tightly linked with
oxidative phosphorylation.
The steps of the cycle include:
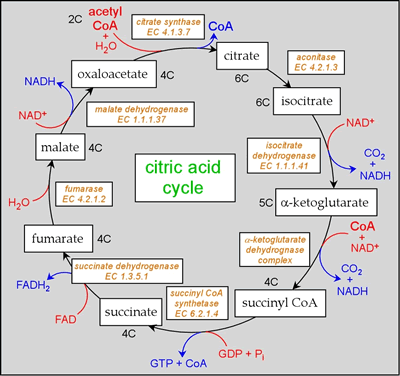
- Citrate synthase [EC 2.3.3.1]:
- Aconitase [EC 4.2.1.3]: Isomerization of citrate to isocitrate
- Isocitrate dehydrogenase [IDH; EC 1.1.1.41]
- α-ketoglutarate dehydrogenase complex: Oxidative decarboxylation of α-ketoglutarate yields succinyl CoA.
- Succinyl CoA synthetase [EC 6.2.1.4]
- Regeneration of oxaloacetate: Succinate dehydrogenase [EC 1.3.5.1], fumarase [EC 4.2.1.2], and malate dehydrogenate [EC 1.1.1.37].
Here, a closer look at the individual reactions and the enzymes that catalyze them, their mechanisms (including roles of cofactors), energetics, and regulation.
Overview of the citric acid cycle
Also known for historical reasons as the tricarboxylic acid cycle or the Krebs cycle, the centrality of the citric acid cycle to oxidative intermediary metabolism is unparalleled. It results in the oxidative (catabolic) processing of acetyl CoA derived from fatty acid and amino acid catabolism, in addition to that from glucose and other carbohydrates. The reduced products of the cycle, NADH and FADH2, are the electron donors for the electron transport chain. The tight linkage between the citric acid cycle and oxidative phosphorylation, called respiratory control, insures that NADH production by the cycle is regulated by the need for energy in the form of ATP. In eukaryotic cells, the citric acid cycle is carried out in mitochondria.
Other than its role in the citric acid cycle, oxaloacetate may also be used to generate glucose by gluconeogenesis, and among citric acid cycle intermediates, oxaloacetate is not alone in having an amphibolic role. This is discussed further below.
One turn of the cycle results in the oxidation of a two-carbon unit. The two-carbon units, in the form of acetyl CoA, enter the cycle by combining with oxaloacetate, in a reaction catalyzed by citrate synthase. The cycle continues with isomerization of citrate to isocitrate, followed by two oxidative decarboxylations, The carbon dioxide given off originates from oxaloacetate, so the release of carbon atoms as CO2 from the added acetyl group occurs in subsequent turns of the cycle. Hydrolysis of succinyl CoA is linked to phosphorylation of GDP (an example of the so-called substrate-level phosphorylation means of capturing the energy released from catabolic processes in the form of high-energy phosphates). Oxaloacetate is then regenerated from succinate in three steps, a FAD-linked dehydrogenation, addition of water, and a final NAD-linked oxidation of a secondary alcohol to a ketone. A stoichiometric equation can be written for one turn of the cycle:
3 NAD+ + FAD + GDP + Pi + acetyl CoA + 2 H2O → 3 NADH + FADH2 + GTP + CoA + 2 CO2
The α-ketoglutarate dehydrogenase complex
The α-ketoglutarate dehydrogenase complex carries out the oxidative decarboxylation process that generates succinyl CoA from α-ketoglutarate. The α-ketoglutarate dehydrogenase complex shows a close resemblance to PDH complex. Like PDH complex, α-ketoglutarate dehydrogenase complex is comprised of three distinct enzymatic subunits performing four activities, and utilizing five different cofactors.
Steps of the α-ketoglutarate dehydrogenase complex:
(1) decarboxylation (E1, formation of hydroxybutyryl-TPP)
(2) oxidation (transfer of succinyl group to lipoamide)
(3) transfer of acetyl group from succinyllipoamide to CoA)
(4) oxidation of dihydrolipoamide to lipoamide (E3, FAD, NAD+)
In addition, the flux through the citric acid cycle is regulated, in part, by responsiveness of α-ketoglutarate dehydrogenase complex activity to allosteric effects, substrate availability, and product inhibition by NADH.
Anaplerotic reactions and the amphibolic nature of the citric acid cycle. To keep the citric acid cycle running (i.e. to maintain continuous flux through the cycle), oxaloacetate must be kept in balance with the entry of acetyl CoA. Intermediates of the citric acid cycle serve as precursors for various biosynthetic products. For example, succinyl CoA is the starting material for heme biosynthesis. The intermediates α-ketoglutarate and oxaloacetate can undergo transamination to form glutamate and aspartate, respectively. Oxaloacetate, as noted above, can be readily siphoned off into gluconeogenesis. This "bleeding off" of amphibolic intermediates is what makes the activity of (catalysis of an anaplerotic reaction) essential to the citric acid cycle, Pyruvate carboxylase is an energy-requiring reaction (driven by the input of ATP) that generates oxaloacetate from pyruvate and bicarbonate, and its activity is thus able to maintain adequate levels of oxaloacetate.
Glyoxylate cycle
In plants and certain microorganisms, acetyl CoA can be reformed into succinate The net synthesis of succinate from two molecules of acetyl CoA takes place in what is called the glyoxylate cycle The cycle formally uses three enzymes from the citric acid cycle, citrate synthase, aconitase, and malate dehydrogenase. The enzymes isocitrate lyase [EC 4.1.3.1] and malate synthase [EC 2.3.3.9] are unique to the cycle.