Lecture 8. Protein structure
Tuesday 12 February 2019
Three-dimensional structure of proteins (continued). Tertiary and quaternary protein structure. Example protein structures. Fibrous protein structure: The α-helical coiled coil and keratin.
Reading: Lehninger - Ch.4, pp.115-142.
Summary
Reading summary. §4.3 Protein tertiary and quaternary structures. Fibrous proteins are adapted for a structural function. α-keratin, collagen. Box 4-2 Permanent waving is biochemical engineering. Box 4-3 -Medicine- (why everyone should eat fresh fruits and vegetables) Silk fibroin. Structural diversity reflects functional diversity in globular proteins. Box 4-4 The Protein Data Bank. Globular proteins have a variety of tertiary structures. Motifs, supersecondary structure. Box 4-6 -Methods- methods for determining the three-dimensional structure of a protein (pp.134-136). Domains. Rules derived from studies of common protein folding patterns. Some proteins or protein segments are intrinsically disordered. Protein motifs are the basis for protein structural classification. Topology diagrams. Protein quaternary structures range from simple dimers to large complexes.
***
Structural hierarchy: Recall the hierarchical description of protein structure: Primary structure relates to the covalent description of a (possibly modified) polypeptide. We have considered the common secondary strucure patterns that are defined mainly by local, repeitive main chain conformations and the hydrogen bonding interactions formed by the main chain polar groups. Here we examine the more global levels of protein structure that involve entire polypeptide chains and associations between separate chains.
Tertiary structure is the overall shape of the polypeptide chain, which depends on the conformation of all the main chain and side chain bonds of the molecule. Quaternary structure refers to the cases in which separate polypeptide chains ("monomers" or "subunits") associate - usually noncovalently - to form "multimers" or "oligomers".
Exploring protein structures. A wealth of protein structure information is readily accessible through the Protein Data Bank (PDB). There are a variety of tools for visualizing and manipulating the structures found there. The PyMOL molecular graphics program is an especially useful and powerful tool, and the images of protein structure representations seen on this website were produced using it.
Keratin
Two important examples of fibrous proteins, which tend to perform structural roles, are keratin and collagen. Let us first examine keratin, which is based on the coiled-coil motif just introduced above. The keratin polypeptide sequence features long stretches of heptad repeats. As a result, two keratin polypeptide chains form α helices that wrap around each other, the axis of each forming a left-handed supercoil. The extended coiled-coils of keratin form higher order structures. This includes cross-linking of cysteine residues via disulfide bonds.
Collagen is a triple helix
Collagen is the principal protein in connective and other fibrous tissues, and is in fact the most abundant protein in humans. The primary structure of the collagen triple helix consists of a repetitive sequence, Gly-X-Y, where X and Y are often Pro or Hyp (hydroxyproline). This forms a left-handed helix with about three residues per turn, and we emphasize that this is not to be confused with the α helix ! Three such left-handed helical chains wind around each other, the axis of each tracing out a right-handed supercoil. The amide group of glycine and acyl oxygen of proline form interchain hydrogen bonds through the middle of the triple helix.
There are many forms of collagen, and higher-order structures, in which triple-helices (denoted as "microfibrils") assemble.
Posttranslational modifications are an especially interesting aspect to collagen. Two important modifications:
- Hydroxyproline (Hyp) - stabilizes the triple helix conformation of collagen
- Allysine residues are modified lysine residues which lead to covalent cross-linking between triple helices
The hydroxylation of proline and lysine residues of collagen is carried out by hydroxylation enzymes that rely on ascorbate (vitamin C) for their function. Absence of vitamin C from the diet of humans leads to the collagen defects underlying the disease scurvy.
Building up tertiary and quaternary structure
The β-α-β motif described previously suggests that one way of viewing protein tertiary structure is as a pattern of connecting together secondary structure features. The simplest such motifs - like β-hairpins, β-α units, helix-turn-helix - occur quite commonly in tertiary structures, and so we can begin to recognize how motifs can be elaborated and assembled into complete tertiary structures.
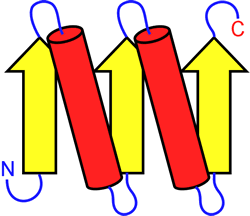
Left: Example of a topology diagram for a polypeptide chain. The parts of the chain that adopt β-strand conformation are shown as yellow arrows, α-helices are shown as red cylinders. These secondary structure elements are connected by the blue loops
Another level of the protein structure hierarchy, quaternary structure, describes how polypeptide chains with well-defined tertiary structures associate together in specific, and most often symmetric, forms. Proteins that adopt quaternary structural forms can have functional advantages over proteins with the same basic activity but no quaternary associations (i.e. those existing as unassociated molecules, or monomers). In fact, the association of α helices into coiled-coil structures like keratin is an example of quaternary structure, and the left-handed superhelical twist of this association gives keratin an extra measure of tensile strength appropriate for this fibrous, structural protein. We'll consider such associations of globular polypeptide chains, as well as the functional properties that arise from these quaternary structures. Principal examples include hemoglobin and aspartate transcarbamoylase (ATCase).
General features of tertiary structure
We can summarize much of what has been learned about protein structure in the form of some generalizations that describe features common to most if not all proteins.
- Proteins have well-packed, non-polar interiors, whereas polar and charged groups are accessible to the outside. Where polar groups occur in the interior (e.g. main chain carbonyls and amides), they tend to be paired.
- The interior of proteins are close-packed, i.e. there are no large cavities.
- The protein chains do not generally form "knots" (although see Taylor & Lin (2003).
- In comparing proteins, it is found that sequence (1° structure) similarity usually implies structural similarity. Note that there are numerous instances in which two proteins with little or no detectable sequence similarity nonetheless adopt very similar structures.
- Overall, there is very little strain in protein structures. In cases where local strain exists, the energetic cost is covered by numerous favorable interactions within the rest of the structure.
.gif)
.gif)
Related topics pages:
- secondary protein structure
- tertiary protein structure
- collagen
- myoglobin
- quaternary structure